Abstract
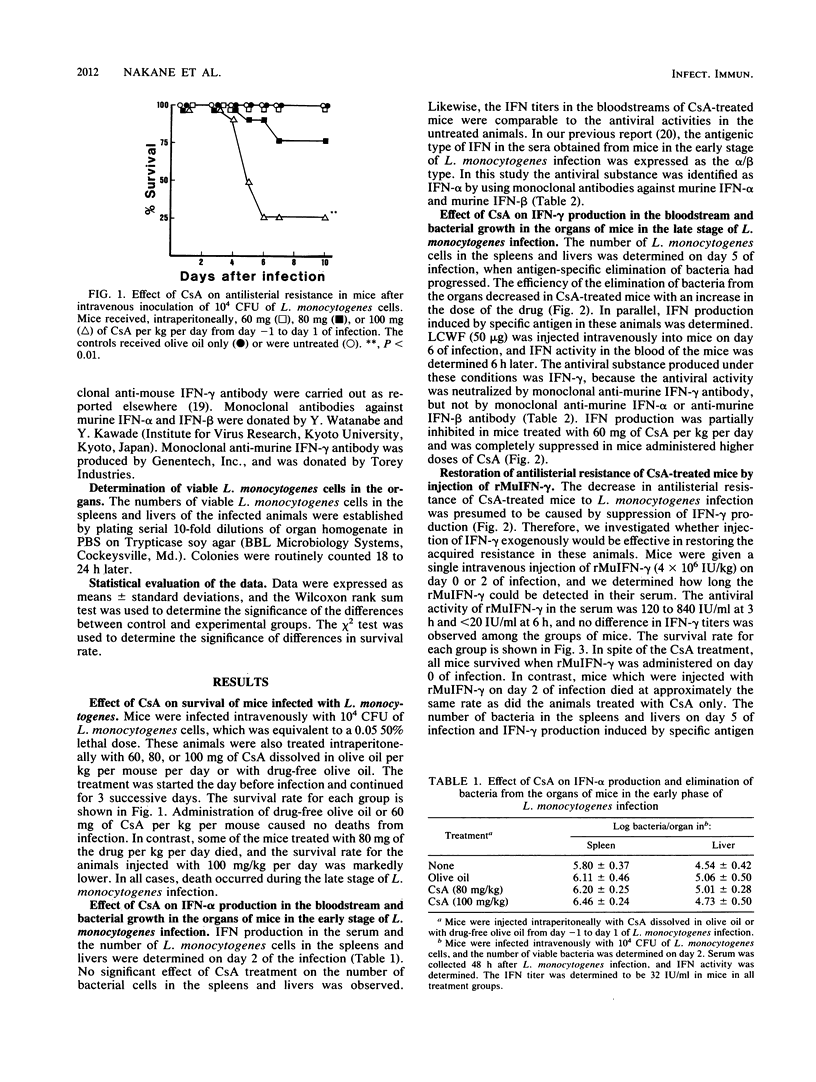
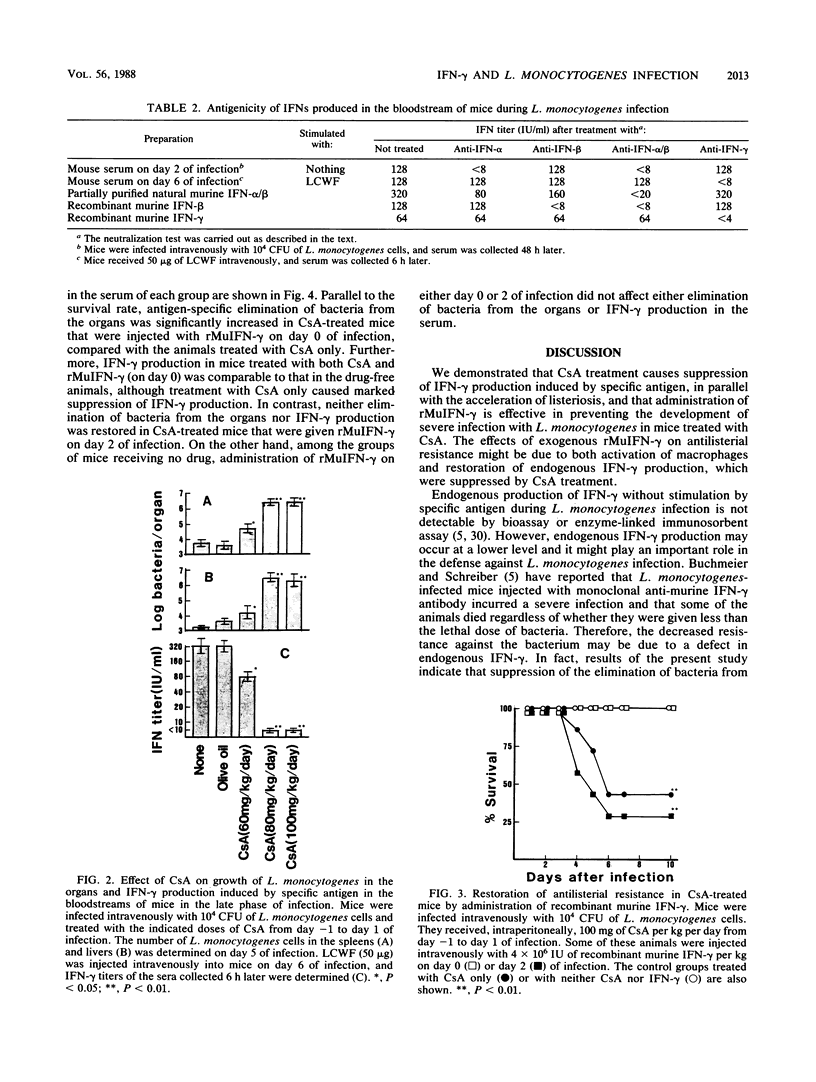
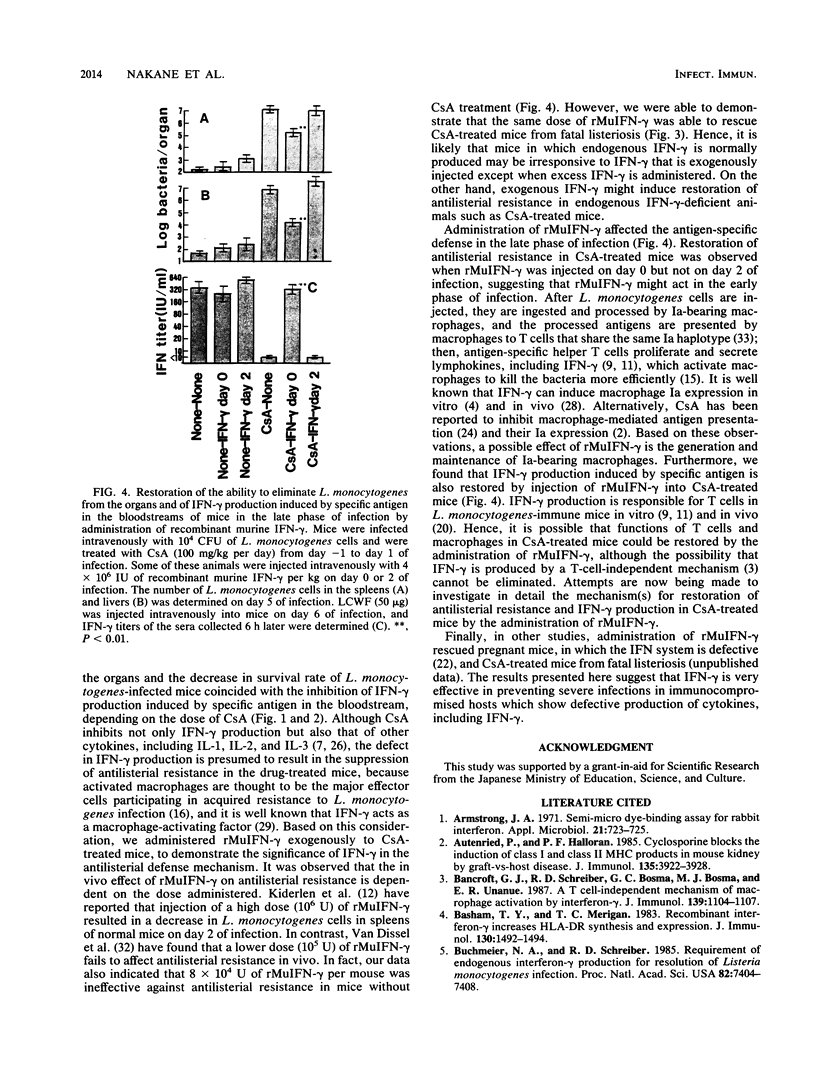
The significance of interferons (IFNs) induced by Listeria monocytogenes in the antilisterial defense mechanism was studied in mice. Cyclosporin A (CsA) had no effect on IFN-alpha production that was induced in the bloodstream after intravenous infection of mice with L. monocytogenes, whereas IFN-gamma that was induced in the bloodstreams of control mice 6 h after stimulation with specific antigen in the late phase of infection was suppressed in CsA-treated mice, depending on the dose of the drug injected. The decrease in IFN-gamma production caused an increase in bacterial growth in the spleens and livers of CsA-treated mice. Furthermore, administration of a daily dose of CsA at 80 or 100 mg/kg of body weight resulted in fatal listeriosis, even though the dose was nonlethal for normal mice. The administration of recombinant murine IFN-gamma on day 0 of L. monocytogenes infection prevented CsA-treated mice from developing fatal listeriosis and restored their ability to produce IFN-gamma in the bloodstream, in response to specific antigen in the late phase of infection.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong J. A. Semi-micro, dye-binding assay for rabbit interferon. Appl Microbiol. 1971 Apr;21(4):723–725. doi: 10.1128/am.21.4.723-725.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autenried P., Halloran P. F. Cyclosporine blocks the induction of class I and class II MHC products in mouse kidney by graft-vs-host disease. J Immunol. 1985 Dec;135(6):3922–3928. [PubMed] [Google Scholar]
- Bancroft G. J., Schreiber R. D., Bosma G. C., Bosma M. J., Unanue E. R. A T cell-independent mechanism of macrophage activation by interferon-gamma. J Immunol. 1987 Aug 15;139(4):1104–1107. [PubMed] [Google Scholar]
- Basham T. Y., Merigan T. C. Recombinant interferon-gamma increases HLA-DR synthesis and expression. J Immunol. 1983 Apr;130(4):1492–1494. [PubMed] [Google Scholar]
- Buchmeier N. A., Schreiber R. D. Requirement of endogenous interferon-gamma production for resolution of Listeria monocytogenes infection. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7404–7408. doi: 10.1073/pnas.82.21.7404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calne R. Y. Immunosuppression for organ grafting -- observations on cyclosporin A. Immunol Rev. 1979;46:113–124. doi: 10.1111/j.1600-065x.1979.tb00286.x. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A., Andrus L., Steinman R. M. Lymphokine and nonlymphokine mRNA levels in stimulated human T cells. Kinetics, mitogen requirements, and effects of cyclosporin A. J Exp Med. 1986 Apr 1;163(4):922–937. doi: 10.1084/jem.163.4.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Cloning and expression of murine immune interferon cDNA. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5842–5846. doi: 10.1073/pnas.80.19.5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havell E. A., Spitalny G. L., Patel P. J. Enhanced production of murine interferon gamma by T cells generated in response to bacterial infection. J Exp Med. 1982 Jul 1;156(1):112–127. doi: 10.1084/jem.156.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügin A. W., Cerny A., Wrann M., Hengartner H., Zinkernagel R. M. Effect of cyclosporin A on immunity to Listeria monocytogenes. Infect Immun. 1986 Apr;52(1):12–17. doi: 10.1128/iai.52.1.12-17.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann S. H., Hahn H. Biological functions of t cell lines with specificity for the intracellular bacterium Listeria monocytogenes in vitro and in vivo. J Exp Med. 1982 Jun 1;155(6):1754–1765. doi: 10.1084/jem.155.6.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiderlen A. F., Kaufmann S. H., Lohmann-Matthes M. L. Protection of mice against the intracellular bacterium Listeria monocytogenes by recombinant immune interferon. Eur J Immunol. 1984 Oct;14(10):964–967. doi: 10.1002/eji.1830141019. [DOI] [PubMed] [Google Scholar]
- Kunkl A., Klaus G. G. Selective effects of cyclosporin A on functional B cell subsets in the mouse. J Immunol. 1980 Dec;125(6):2526–2531. [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama M., Takeya K., Nomoto K., Shimotori S. Three phases of phagocyte contribution to resistance against Listeria monocytogenes. J Gen Microbiol. 1978 May;106(1):165–171. doi: 10.1099/00221287-106-1-165. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T. Alternative induction of IFN-alpha and IFN-gamma by Listeria monocytogenes in human peripheral blood mononuclear leukocyte cultures. J Immunol. 1981 Jun;126(6):2139–2142. [PubMed] [Google Scholar]
- Nakane A., Minagawa T. Alternative induction of alpha/beta interferons and gamma interferon by listeria monocytogenes in mouse spleen cell cultures. Cell Immunol. 1983 Feb 1;75(2):283–291. doi: 10.1016/0008-8749(83)90326-x. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T. Induction of alpha and beta interferons during the hyporeactive state of gamma interferon by Mycobacterium bovis BCG cell wall fraction in Mycobacterium bovis BCG-sensitized mice. Infect Immun. 1982 Jun;36(3):966–970. doi: 10.1128/iai.36.3.966-970.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T. Sequential production of alpha and beta interferons and gamma interferon in the circulation of Listeria monocytogenes-infected mice after stimulation with bacterial lipopolysaccharide. Microbiol Immunol. 1985;29(7):659–669. doi: 10.1111/j.1348-0421.1985.tb00869.x. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T. The significance of alpha/beta interferons and gamma interferon produced in mice infected with Listeria monocytogenes. Cell Immunol. 1984 Oct 1;88(1):29–40. doi: 10.1016/0008-8749(84)90049-2. [DOI] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Yasuda I. Induction of alpha/beta interferon and gamma interferon in mice infected with Listeria monocytogenes during pregnancy. Infect Immun. 1985 Dec;50(3):877–880. doi: 10.1128/iai.50.3.877-880.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palay D. A., Cluff C. W., Wentworth P. A., Ziegler H. K. Cyclosporine inhibits macrophage-mediated antigen presentation. J Immunol. 1986 Jun 15;136(12):4348–4353. [PubMed] [Google Scholar]
- Rullan P. P., Barr R. J., Cole G. W. Cyclosporine and murine allergic contact dermatitis. Arch Dermatol. 1984 Sep;120(9):1179–1183. [PubMed] [Google Scholar]
- Schaffner A., Douglas H., Davis C. E. Models of T cell deficiency in listeriosis: the effects of cortisone and cyclosporin A on normal and nude BALB/c mice. J Immunol. 1983 Jul;131(1):450–453. [PubMed] [Google Scholar]
- Schreiber R. D., Hicks L. J., Celada A., Buchmeier N. A., Gray P. W. Monoclonal antibodies to murine gamma-interferon which differentially modulate macrophage activation and antiviral activity. J Immunol. 1985 Mar;134(3):1609–1618. [PubMed] [Google Scholar]
- Shevach E. M. The effects of cyclosporin A on the immune system. Annu Rev Immunol. 1985;3:397–423. doi: 10.1146/annurev.iy.03.040185.002145. [DOI] [PubMed] [Google Scholar]
- Shidani B., Milon G., Marchal G., Truffa-Bachi P. Cyclosporin A inhibits the delayed-type hypersensitivity reaction: impaired production of early pro-inflammatory mediator(s). Eur J Immunol. 1984 Apr;14(4):314–318. doi: 10.1002/eji.1830140407. [DOI] [PubMed] [Google Scholar]
- Skoskiewicz M. J., Colvin R. B., Schneeberger E. E., Russell P. S. Widespread and selective induction of major histocompatibility complex-determined antigens in vivo by gamma interferon. J Exp Med. 1985 Nov 1;162(5):1645–1664. doi: 10.1084/jem.162.5.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R., Heymer B., Hof H. Effects of cyclosporin A on experimental infection with Listeria monocytogenes. Clin Exp Immunol. 1985 Dec;62(3):491–498. [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]
- van Dissel J. T., Stikkelbroeck J. J., Michel B. C., van den Barselaar M. T., Leijh P. C., van Furth R. Inability of recombinant interferon-gamma to activate the antibacterial activity of mouse peritoneal macrophages against Listeria monocytogenes and Salmonella typhimurium. J Immunol. 1987 Sep 1;139(5):1673–1678. [PubMed] [Google Scholar]


