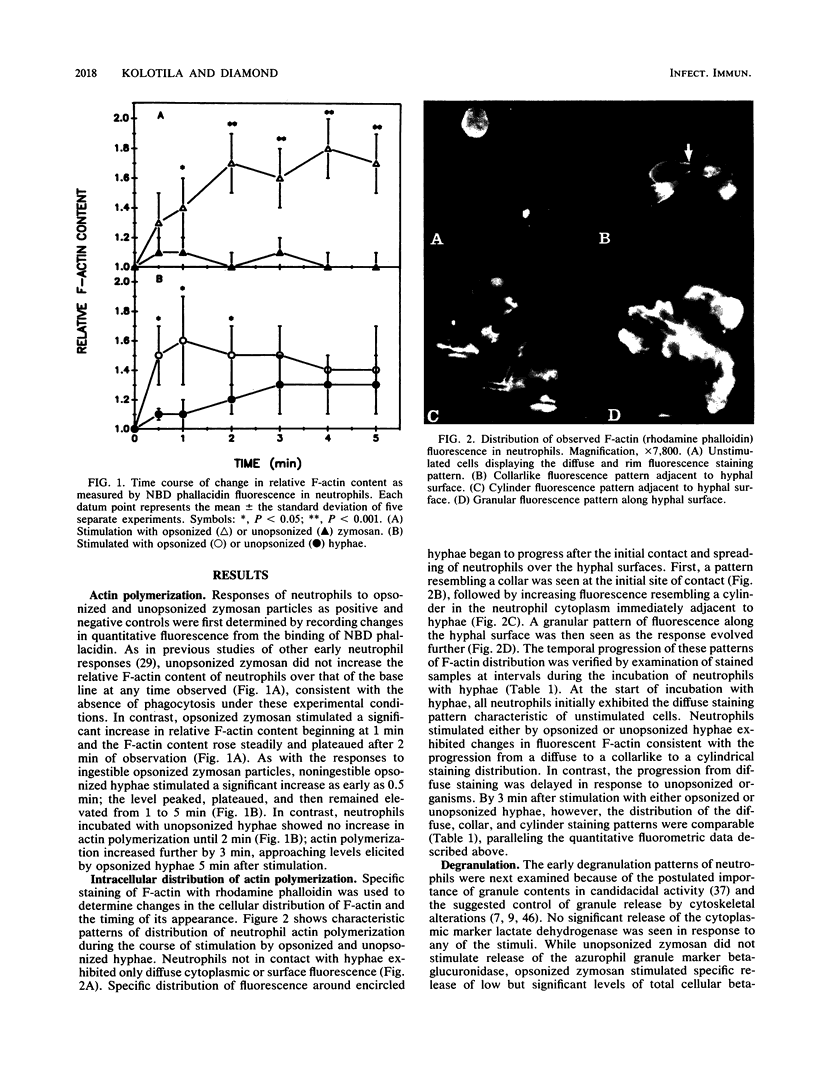
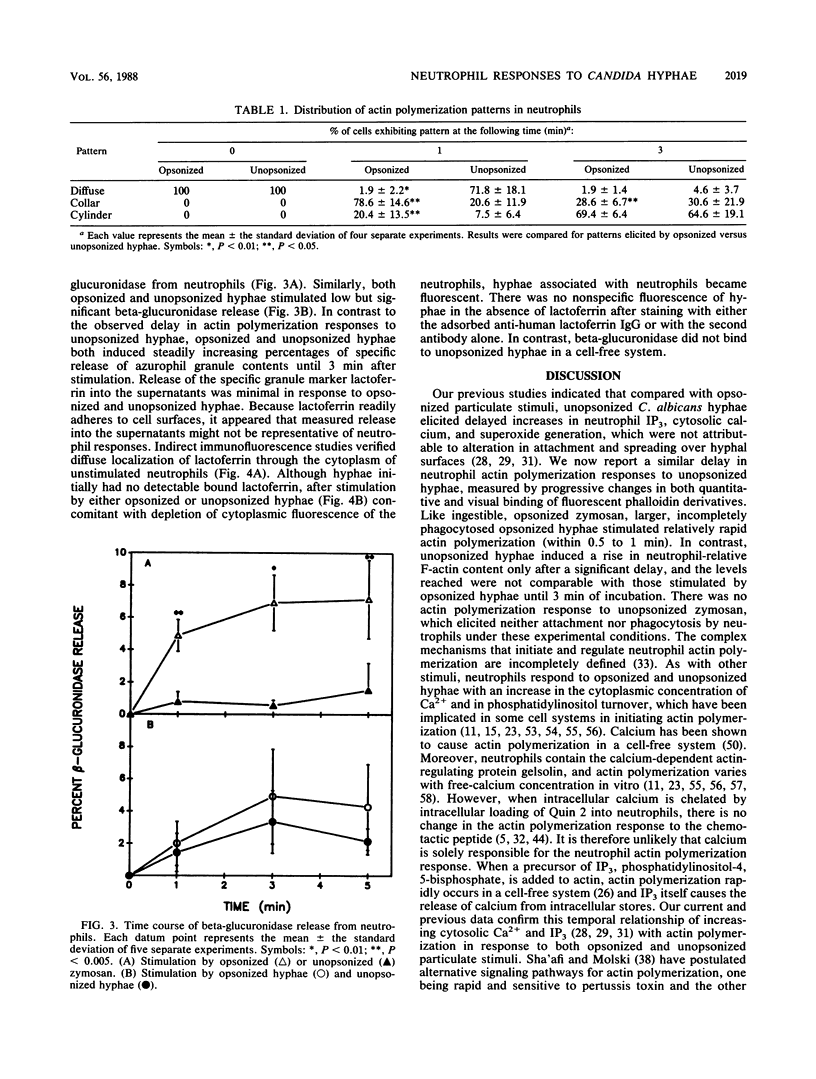
Abstract
We previously showed that unopsonized Candida albicans hyphae stimulated a delayed rise in the putative neutrophil second messengers Ca2+ and inositol 1,4,5-trisphosphate and subsequent O2- release, as compared with opsonized hyphae or zymosan. Therefore, cytoskeletal and degranulation temporal responses to these stimuli were examined. Unopsonized zymosan elicited no neutrophil responses under the experimental condition used. Neutrophil actin polymerization (quantitated by fluorescent measurements of NBD phallacidin) was rapid after stimulation by opsonized hyphae or zymosan (peaking at 1 and 2 min, respectively). This corresponded to observed changes in microscopic actin polymerization, measured with rhodamine phalloidin, which progressed from initially diffuse to collarlike to cylinderlike staining patterns surrounding the hyphae. Compared with opsonized hyphae, unopsonized hyphae resulted in a delayed appearance of the last two visible patterns (P less than 0.05) and in quantitative actin polymerization despite similarly rapid initial contact and spreading over the hyphae by neutrophils. Unlike other neutrophil responses, degranulation did not follow the delayed patterns of responses to stimulation with unopsonized hyphae. In the absence of the release of the cytoplasmic marker lactate dehydrogenase, the release of beta-glucuronidase, an azurophil granule marker, gradually and progressively rose in response to all of the stimuli but unopsonized zymosan. The low but significant levels observed were within a range consistent with published results for degranulation responses to particulate stimuli without cytochalasin B. A quantitative immunoassay of lactoferrin, a specific granule marker, detected no release into supernatants, and immunofluorescent staining indicated concomitant depletion of lactoferrin from neutrophil granules and binding to hyphal and neutrophil surfaces after stimulation by unopsonized hyphae. Thus, the delayed actin polymerization response to unopsonized hyphae occurred subsequent to neutrophil attachment and spreading and resembled the temporal sequence of other neutrophil responses linked to the respiratory burst. In contrast, the degranulation responses to all stimuli appeared to begin and progress gradually after observed attachment and spreading of the neutrophil over hyphal surfaces without a clear temporal relationship to rises in cytoplasmic Ca2+ or F-actin. In addition, the avid binding of released lactoferrin to cell surfaces eliminates its value as a quantitative marker of enzyme release but raises the possibility that it might participate in fungicidal activity.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
- Ambruso D. R., Johnston R. B., Jr Lactoferrin enhances hydroxyl radical production by human neutrophils, neutrophil particulate fractions, and an enzymatic generating system. J Clin Invest. 1981 Feb;67(2):352–360. doi: 10.1172/JCI110042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold R. R., Brewer M., Gauthier J. J. Bactericidal activity of human lactoferrin: sensitivity of a variety of microorganisms. Infect Immun. 1980 Jun;28(3):893–898. doi: 10.1128/iai.28.3.893-898.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin D. A., Jenny E. R., Aisen P. The effect of human serum transferrin and milk lactoferrin on hydroxyl radical formation from superoxide and hydrogen peroxide. J Biol Chem. 1984 Nov 10;259(21):13391–13394. [PubMed] [Google Scholar]
- Bengtsson T., Stendahl O., Andersson T. The role of the cytosolic free Ca2+ transient for fMet-Leu-Phe induced actin polymerization in human neutrophils. Eur J Cell Biol. 1986 Dec;42(2):338–343. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Boyles J., Bainton D. F. Changes in plasma-membrane-associated filaments during endocytosis and exocytosis in polymorphonuclear leukocytes. Cell. 1981 Jun;24(3):905–914. doi: 10.1016/0092-8674(81)90116-1. [DOI] [PubMed] [Google Scholar]
- Britigan B. E., Rosen G. M., Thompson B. Y., Chai Y., Cohen M. S. Stimulated human neutrophils limit iron-catalyzed hydroxyl radical formation as detected by spin-trapping techniques. J Biol Chem. 1986 Dec 25;261(36):17026–17032. [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R. Reorganisation of peripheral actin filaments as a prelude to exocytosis. Biosci Rep. 1987 Apr;7(4):281–288. doi: 10.1007/BF01121449. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Borregaard N. Neutrophils autoinactivate secretory products by myeloperoxidase-catalyzed oxidation. Blood. 1985 Feb;65(2):375–381. [PubMed] [Google Scholar]
- Daimond R. D., Krzesicki R. Mechanisms of attachment of neutrophils to Candida albicans pseudohyphae in the absence of serum, and of subsequent damage to pseudohyphae by microbicidal processes of neutrophils in vitro. J Clin Invest. 1978 Feb;61(2):360–369. doi: 10.1172/JCI108946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancker P., Löw I. Dual effect of Ca2+ on ultrasonic ATPase activity and polymerization of muscle actin. Biochim Biophys Acta. 1977 Sep 15;484(1):169–176. doi: 10.1016/0005-2744(77)90122-x. [DOI] [PubMed] [Google Scholar]
- Diamond R. D., Clark R. A., Haudenschild C. C. Damage to Candida albicans hyphae and pseudohyphae by the myeloperoxidase system and oxidative products of neutrophil metabolism in vitro. J Clin Invest. 1980 Nov;66(5):908–917. doi: 10.1172/JCI109958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond R. D., Krzesicki R., Jao W. Damage to pseudohyphal forms of Candida albicans by neutrophils in the absence of serum in vitro. J Clin Invest. 1978 Feb;61(2):349–359. doi: 10.1172/JCI108945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Europe-Finner G. N., Newell P. C. Inositol 1,4,5-trisphosphate and calcium stimulate actin polymerization in Dictyostelium discoideum. J Cell Sci. 1986 Jun;82:41–51. doi: 10.1242/jcs.82.1.41. [DOI] [PubMed] [Google Scholar]
- Ganz T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect Immun. 1987 Mar;55(3):568–571. doi: 10.1128/iai.55.3.568-571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington S. V., Spitznagel J. K., Quie P. G. An enzyme-linked immunoassay (ELISA) for measurement of lactoferrin. J Immunol Methods. 1983 Dec 16;65(1-2):183–190. doi: 10.1016/0022-1759(83)90314-9. [DOI] [PubMed] [Google Scholar]
- Hoffstein S., Weissmann G. Microfilaments and microtubules in calcium ionophore-induced secretion of lysosomal enzymes from human polymorphonuclear leukocytes. J Cell Biol. 1978 Sep;78(3):769–781. doi: 10.1083/jcb.78.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T. H., Oresajo C. O. The kinetics of chemotactic peptide-induced change in F-actin content, F-actin distribution, and the shape of neutrophils. J Cell Biol. 1985 Sep;101(3):1078–1085. doi: 10.1083/jcb.101.3.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T. H., Wang D. Calcium ionophore, phorbol ester, and chemotactic peptide-induced cytoskeleton reorganization in human neutrophils. J Clin Invest. 1987 May;79(5):1359–1364. doi: 10.1172/JCI112962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jesaitis A. J., Naemura J. R., Painter R. G., Sklar L. A., Cochrane C. G. The fate of an N-formylated chemotactic peptide in stimulated human granulocytes. Subcellular fractionation studies. J Biol Chem. 1983 Feb 10;258(3):1968–1977. [PubMed] [Google Scholar]
- Jesaitis A. J., Tolley J. O., Allen R. A. Receptor-cytoskeleton interactions and membrane traffic may regulate chemoattractant-induced superoxide production in human granulocytes. J Biol Chem. 1986 Oct 15;261(29):13662–13669. [PubMed] [Google Scholar]
- KASAI M., ASAKURA S., OOSAWA F. The G-F equilibrium in actin solutions under various conditions. Biochim Biophys Acta. 1962 Feb 12;57:13–21. doi: 10.1016/0006-3002(62)91072-7. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Green I., Rich R. R., Schade A. L. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J Infect Dis. 1971 Dec;124(6):539–544. doi: 10.1093/infdis/124.6.539. [DOI] [PubMed] [Google Scholar]
- Lassing I., Lindberg U. Specific interaction between phosphatidylinositol 4,5-bisphosphate and profilactin. Nature. 1985 Apr 4;314(6010):472–474. doi: 10.1038/314472a0. [DOI] [PubMed] [Google Scholar]
- Leffell M. S., Spitznagel J. K. Fate of human lactoferrin and myeloperoxidase in phagocytizing human neutrophils: effects of immunoglobulin G subclasses and immune complexes coated on latex beads. Infect Immun. 1975 Oct;12(4):813–820. doi: 10.1128/iai.12.4.813-820.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Lyman C. A., Murata T., Sullivan J. A., Mandell G. L., Diamond R. D. Cytosolic calcium changes in individual neutrophils stimulated by opsonized and unopsonized Candida albicans hyphae. Infect Immun. 1987 Nov;55(11):2783–2788. doi: 10.1128/iai.55.11.2783-2788.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman C. A., Simons E. R., Melnick D. A., Diamond R. D. Unopsonized Candida albicans hyphae stimulate a neutrophil respiratory burst and a cytosolic calcium flux without membrane depolarization. J Infect Dis. 1987 Nov;156(5):770–776. doi: 10.1093/infdis/156.5.770. [DOI] [PubMed] [Google Scholar]
- Maallem H., Sheppard K., Fletcher J. The discharge of primary and secondary granules during immune phagocytosis by normal and chronic granulocytic leukaemia polymorphonuclear neutrophils. Br J Haematol. 1982 Jun;51(2):201–208. [PubMed] [Google Scholar]
- Meshulam T., Diamond R. D., Lyman C. A., Wysong D. R., Melnick D. A. Temporal association of calcium mobilization, inositol trisphosphate generation, and superoxide anion release by human neutrophils activated by serum opsonized and nonopsonized particulate stimuli. Biochem Biophys Res Commun. 1988 Jan 29;150(2):532–539. doi: 10.1016/0006-291x(88)90426-3. [DOI] [PubMed] [Google Scholar]
- Meshulam T., Proto P., Diamond R. D., Melnick D. A. Calcium modulation and chemotactic response: divergent stimulation of neutrophil chemotaxis and cytosolic calcium response by the chemotactic peptide receptor. J Immunol. 1986 Sep 15;137(6):1954–1960. [PubMed] [Google Scholar]
- Omann G. M., Swann W. N., Oades Z. G., Parkos C. A., Jesaitis A. J., Sklar L. A. N-formylpeptide-receptor dynamics, cytoskeletal activation, and intracellular calcium response in human neutrophil cytoplasts. J Immunol. 1987 Nov 15;139(10):3447–3455. [PubMed] [Google Scholar]
- Pryzwansky K. B., MacRae E. K., Spitznagel J. K., Cooney M. H. Early degranulation of human neutrophils: immunocytochemical studies of surface and intracellular phagocytic events. Cell. 1979 Dec;18(4):1025–1033. doi: 10.1016/0092-8674(79)90215-0. [DOI] [PubMed] [Google Scholar]
- Pryzwansky K. B., Martin L. E., Spitznagel J. K. Immunocytochemical localization of myeloperoxidase, lactoferrin, lysozyme and neutral proteases in human monocytes and neutrophilic granulocytes. J Reticuloendothel Soc. 1978 Sep;24(3):295–310. [PubMed] [Google Scholar]
- Selsted M. E., Szklarek D., Ganz T., Lehrer R. I. Activity of rabbit leukocyte peptides against Candida albicans. Infect Immun. 1985 Jul;49(1):202–206. doi: 10.1128/iai.49.1.202-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha'afi R. I., Molski T. F. Signalling for increased cytoskeletal actin in neutrophils. Biochem Biophys Res Commun. 1987 Jun 15;145(2):934–941. doi: 10.1016/0006-291x(87)91055-2. [DOI] [PubMed] [Google Scholar]
- Sheterline P., Rickard J. E., Richards R. C. Fc receptor-directed phagocytic stimuli induce transient actin assembly at an early stage of phagocytosis in neutrophil leukocytes. Eur J Cell Biol. 1984 May;34(1):80–87. [PubMed] [Google Scholar]
- Sklar L. A., Jesaitis A. J., Painter R. G., Cochrane C. G. The kinetics of neutrophil activation. The response to chemotactic peptides depends upon whether ligand-receptor interaction is rate-limiting. J Biol Chem. 1981 Oct 10;256(19):9909–9914. [PubMed] [Google Scholar]
- Sklar L. A., McNeil V. M., Jesaitis A. J., Painter R. G., Cochrane C. G. A continuous, spectroscopic analysis of the kinetics of elastase secretion by neutrophils. The dependence of secretion upon receptor occupancy. J Biol Chem. 1982 May 25;257(10):5471–5475. [PubMed] [Google Scholar]
- Sklar L. A., Oades Z. G., Jesaitis A. J., Painter R. G., Cochrane C. G. Fluoresceinated chemotactic peptide and high-affinity antifluorescein antibody as a probe of the temporal characteristics of neutrophil stimulation. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7540–7544. doi: 10.1073/pnas.78.12.7540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar L. A., Oades Z. G. Signal transduction and ligand-receptor dynamics in the neutrophil. Ca2+ modulation and restoration. J Biol Chem. 1985 Sep 25;260(21):11468–11475. [PubMed] [Google Scholar]
- Sklar L. A., Omann G. M., Painter R. G. Relationship of actin polymerization and depolymerization to light scattering in human neutrophils: dependence on receptor occupancy and intracellular Ca++. J Cell Biol. 1985 Sep;101(3):1161–1166. doi: 10.1083/jcb.101.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southwick F. S., Stossel T. P. Contractile proteins in leukocyte function. Semin Hematol. 1983 Oct;20(4):305–321. [PubMed] [Google Scholar]
- Stendahl O. I., Hartwig J. H., Brotschi E. A., Stossel T. P. Distribution of actin-binding protein and myosin in macrophages during spreading and phagocytosis. J Cell Biol. 1980 Feb;84(2):215–224. doi: 10.1083/jcb.84.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talstad I., Dalen H., Lehmann V. Degranulation and enzyme release during phagocytosis of inert particles and of bacteria by polymorphonuclear neutrophil granulocytes. Acta Pathol Microbiol Immunol Scand C. 1983 Dec;91(6):403–411. [PubMed] [Google Scholar]
- Tellam R. Mechanism of CaCl2-induced actin polymerization. Biochemistry. 1985 Jul 30;24(16):4455–4460. doi: 10.1021/bi00337a029. [DOI] [PubMed] [Google Scholar]
- Valerius N. H., Stendahl O., Hartwig J. H., Stossel T. P. Distribution of actin-binding protein and myosin in polymorphonuclear leukocytes during locomotion and phagocytosis. Cell. 1981 Apr;24(1):195–202. doi: 10.1016/0092-8674(81)90515-8. [DOI] [PubMed] [Google Scholar]
- Wallace P. J., Wersto R. P., Packman C. H., Lichtman M. A. Chemotactic peptide-induced changes in neutrophil actin conformation. J Cell Biol. 1984 Sep;99(3):1060–1065. doi: 10.1083/jcb.99.3.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. T., Snyderman R., Pike M. C., Lefkowitz R. J. Specific receptor sites for chemotactic peptides on human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1204–1208. doi: 10.1073/pnas.74.3.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassin R., Shefcyk J., White J. R., Tao W., Volpi M., Molski T. F., Naccache P. H., Feinstein M. B., Sha'afi R. I. Effects of chemotactic factors and other agents on the amounts of actin and a 65,000-mol-wt protein associated with the cytoskeleton of rabbit and human neutrophils. J Cell Biol. 1985 Jul;101(1):182–188. doi: 10.1083/jcb.101.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H. L., Hartwig J. H., Maruyama K., Stossel T. P. Ca2+ control of actin filament length. Effects of macrophage gelsolin on actin polymerization. J Biol Chem. 1981 Sep 25;256(18):9693–9697. [PubMed] [Google Scholar]
- Yin H. L., Stossel T. P. Control of cytoplasmic actin gel-sol transformation by gelsolin, a calcium-dependent regulatory protein. Nature. 1979 Oct 18;281(5732):583–586. doi: 10.1038/281583a0. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Zaner K. S., Stossel T. P. Ca2+ control of actin gelation. Interaction of gelsolin with actin filaments and regulation of actin gelation. J Biol Chem. 1980 Oct 10;255(19):9494–9500. [PubMed] [Google Scholar]