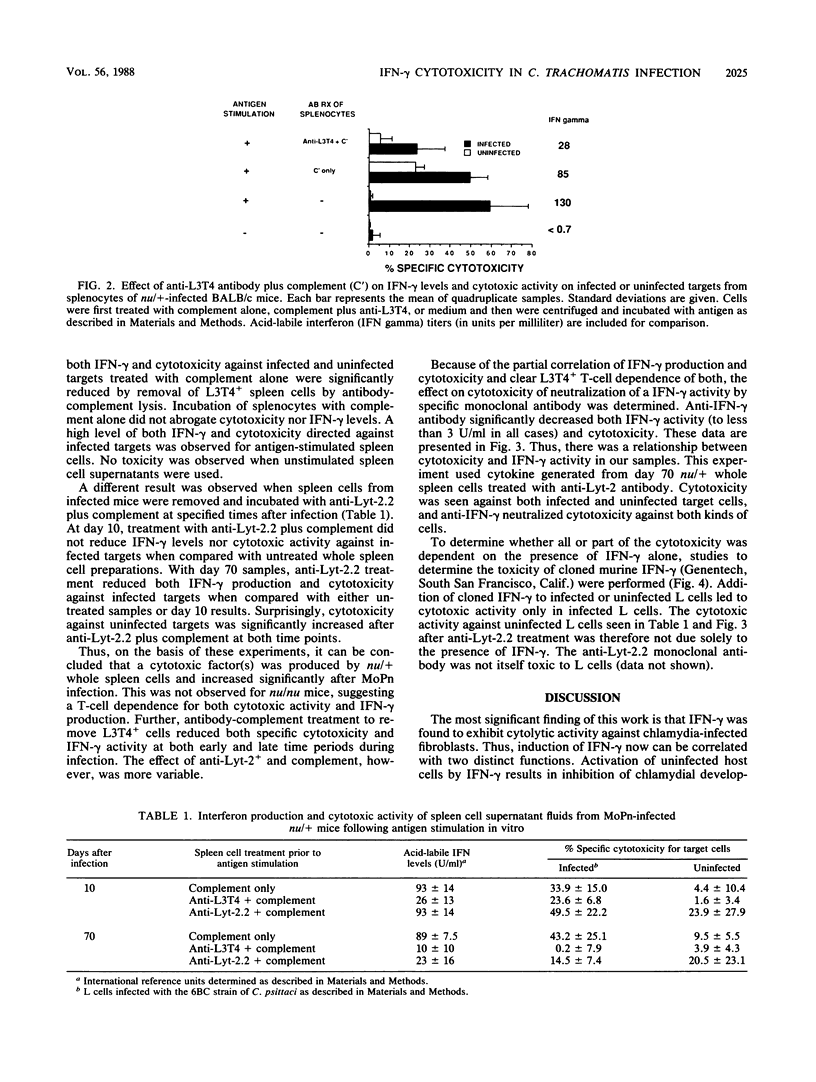
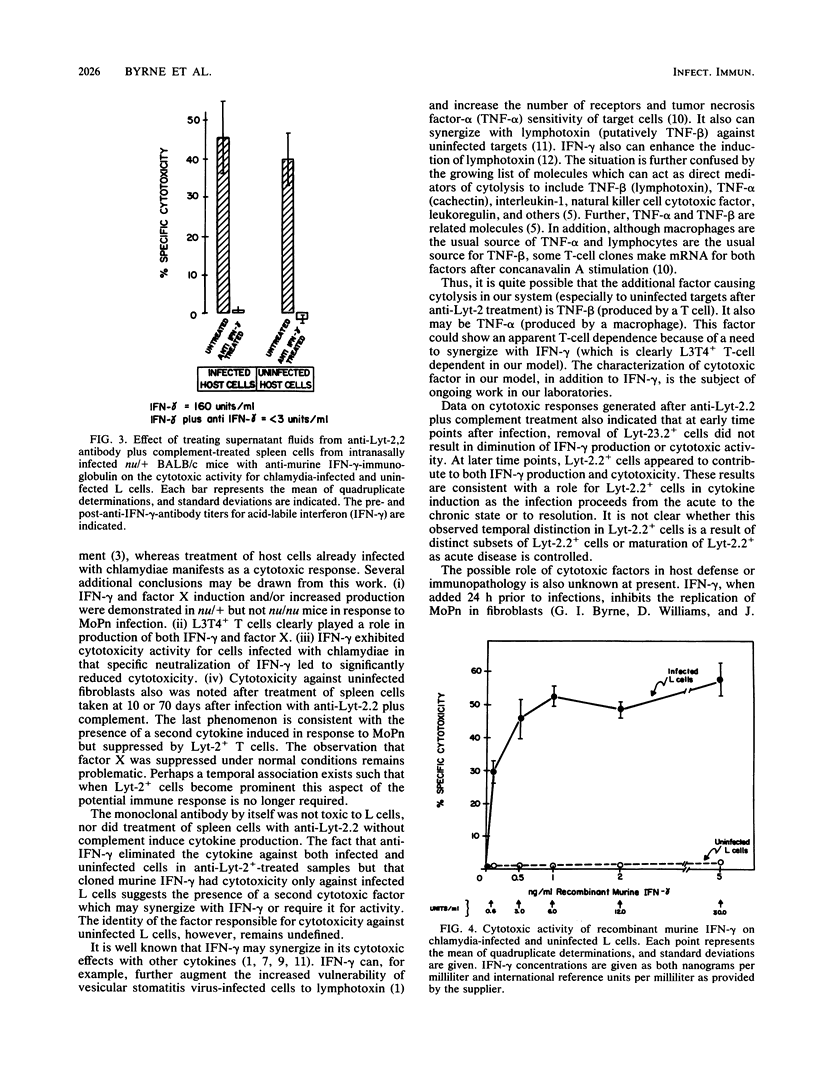
Abstract
After infection with the mouse pneumonitis agent (MoPn; murine Chlamydia trachomatis), heterozygous (nu/+) but not nude athymic (nu/nu) mice produced enhanced amounts of gamma interferon (IFN-gamma) in vitro in response to MoPn antigen that exhibited cytotoxic activity when added to host cells already infected with chlamydiae. Antibody-complement lysis showed the cytotoxic activity to be dependent, at least in part, on L3T4+ T cells for production. The cytotoxic responses were directed primarily against Chlamydia-infected target cells, but a second type of toxicity was demonstrable against uninfected target cells after treatment of the generating cell population with anti-Lyt-2 antibody plus complement at certain time points after infection. This additional nonspecific cytotoxic activity was presumably due to a second factor (factor X) acting in concert with IFN-gamma. Lyt-2+ cells, however, also were shown to play a role in IFN-gamma production and cytotoxicity directed against infected targets at later time points after infection. Neutralization of IFN-gamma in the samples containing cytotoxic activity abrogated the cytotoxicity against both infected and uninfected targets, but cloned murine IFN-gamma exhibited toxicity in a dose-dependent manner only against infected target cells. The data provides evidence that cytotoxicity against infected targets is due to antigen-specific induction of IFN-gamma, but other cytokine activity, most demonstrable after removal of Lyt-2.2+ cells and cytotoxic to uninfected targets, also is present.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Novick D., Hahn T., Fischer D. G., Wallach D. Increase of vulnerability to lymphotoxin in cells infected by vesicular stomatitis virus and its further augmentation by interferon. Cell Immunol. 1985 May;92(2):218–225. doi: 10.1016/0008-8749(85)90003-6. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Grubbs B., Dickey T. J., Schachter J., Williams D. M. Interferon in recovery from pneumonia due to Chlamydia trachomatis in the mouse. J Infect Dis. 1987 Dec;156(6):993–996. doi: 10.1093/infdis/156.6.993. [DOI] [PubMed] [Google Scholar]
- Byrne G. I., Krueger D. A. In vitro expression of factor-mediated cytotoxic activity generated during the immune response to Chlamydia in the mouse. J Immunol. 1985 Jun;134(6):4189–4193. [PubMed] [Google Scholar]
- Lammert J. K. Cytotoxic cells induced after Chlamydia psittaci infection in mice. Infect Immun. 1982 Mar;35(3):1011–1017. doi: 10.1128/iai.35.3.1011-1017.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. H., Aggarwal B. B., Rinderknecht E., Assisi F., Chiu H. The synergistic anti-proliferative effect of gamma-interferon and human lymphotoxin. J Immunol. 1984 Sep;133(3):1083–1086. [PubMed] [Google Scholar]
- Pavia C. S., Schachter J. Failure to detect cell-mediated cytotoxicity against Chlamydia trachomatis-infected cells. Infect Immun. 1983 Mar;39(3):1271–1274. doi: 10.1128/iai.39.3.1271-1274.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell M. B., Conta B. S., Horowitz M., Ruddle N. H. The differential inhibitory effect of lymphotoxin and immune interferon on normal and malignant lymphoid cells. Lymphokine Res. 1985 Winter;4(1):13–26. [PubMed] [Google Scholar]
- Stone-Wolff D. S., Yip Y. K., Kelker H. C., Le J., Henriksen-Destefano D., Rubin B. Y., Rinderknecht E., Aggarwal B. B., Vilcek J. Interrelationships of human interferon-gamma with lymphotoxin and monocyte cytotoxin. J Exp Med. 1984 Mar 1;159(3):828–843. doi: 10.1084/jem.159.3.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svedersky L. P., Nedwin G. E., Goeddel D. V., Palladino M. A., Jr Interferon-gamma enhances induction of lymphotoxin in recombinant interleukin 2-stimulated peripheral blood mononuclear cells. J Immunol. 1985 Mar;134(3):1604–1608. [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Coalson J. J., Grubbs B. Cellular immunity to the mouse pneumonitis agent. J Infect Dis. 1984 Apr;149(4):630–639. doi: 10.1093/infdis/149.4.630. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Drutz D. J., Sumaya C. V. Pneumonia due to Chlamydia trachomatis in the immunocompromised (nude) mouse. J Infect Dis. 1981 Feb;143(2):238–241. doi: 10.1093/infdis/143.2.238. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Schachter J., Grubbs B., Sumaya C. V. The role of antibody in host defense against the agent of mouse pneumonitis. J Infect Dis. 1982 Feb;145(2):200–205. doi: 10.1093/infdis/145.2.200. [DOI] [PubMed] [Google Scholar]


