Abstract
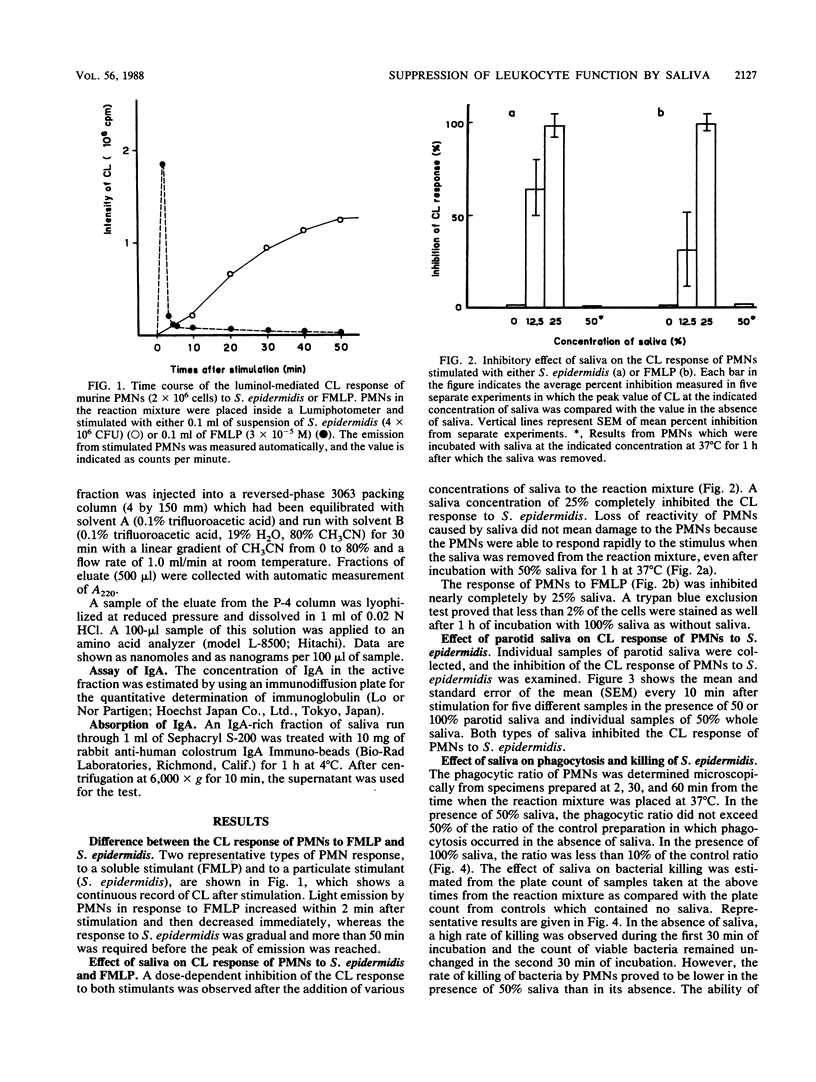
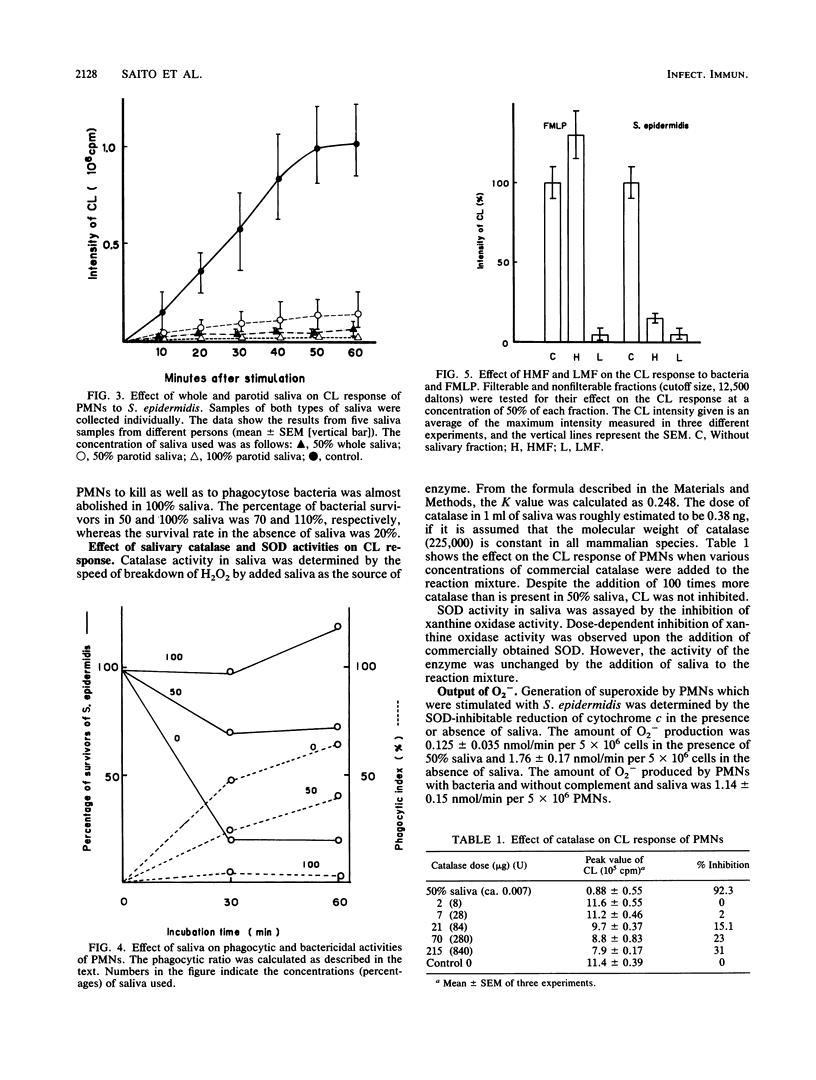
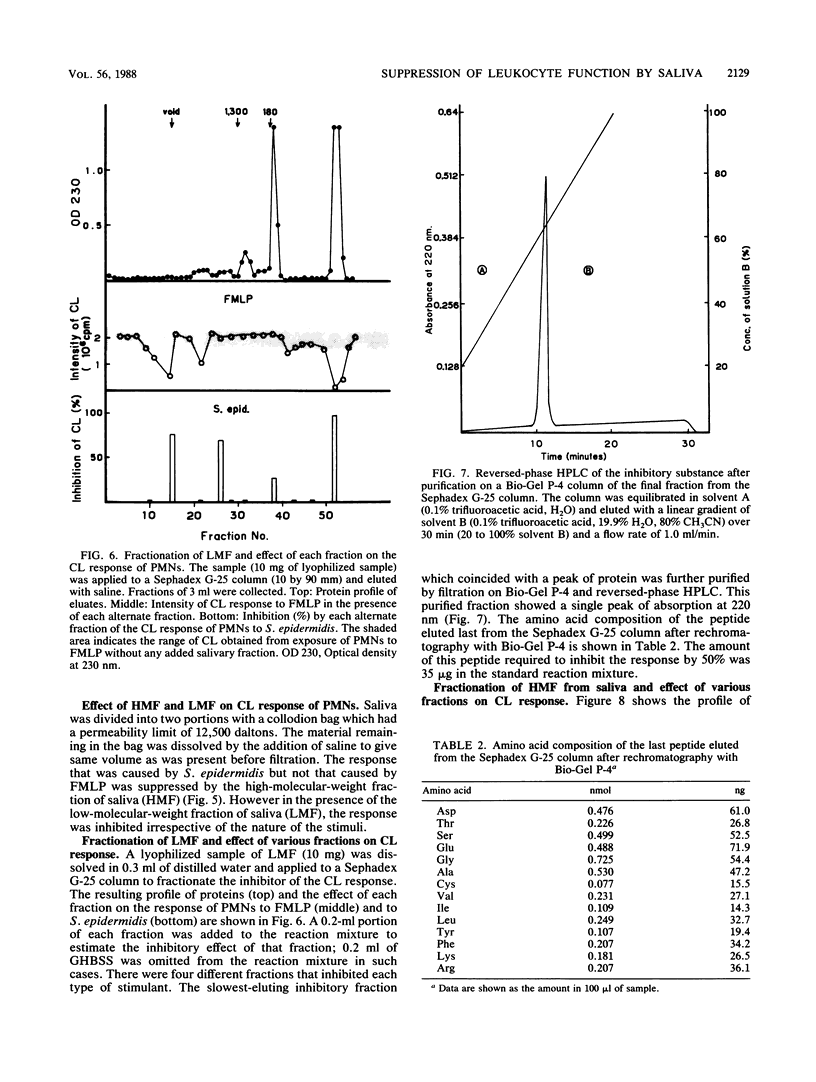
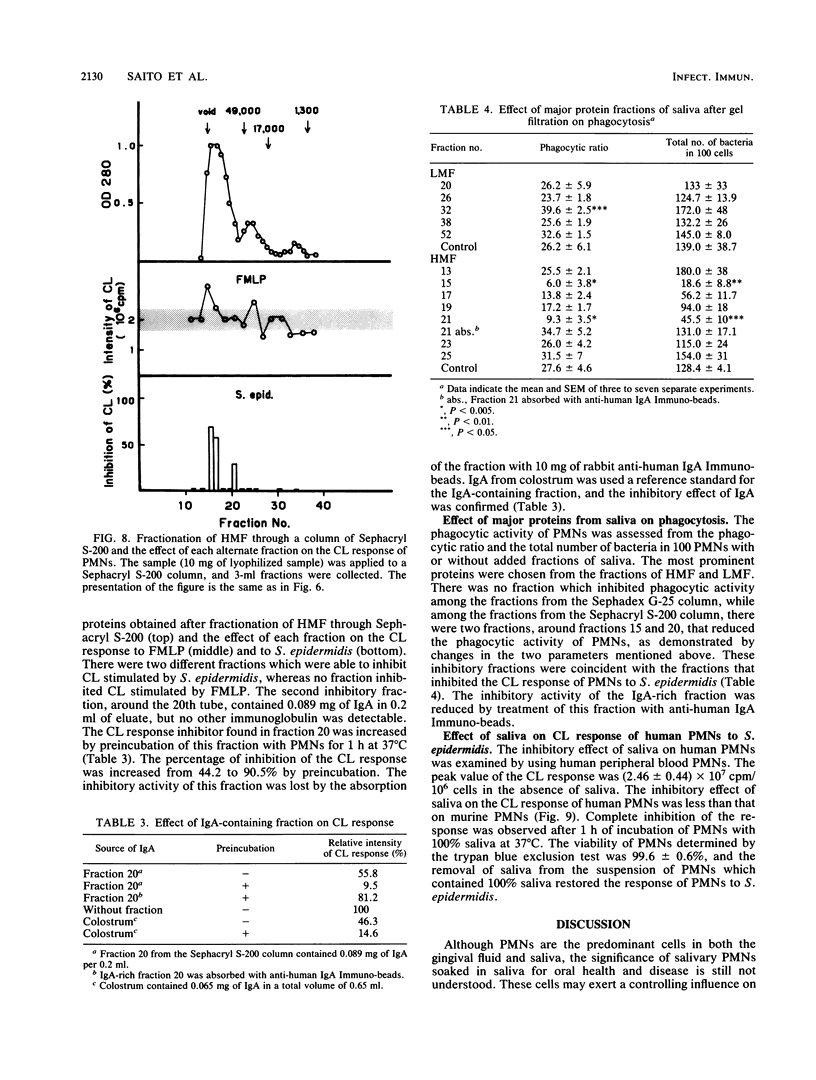
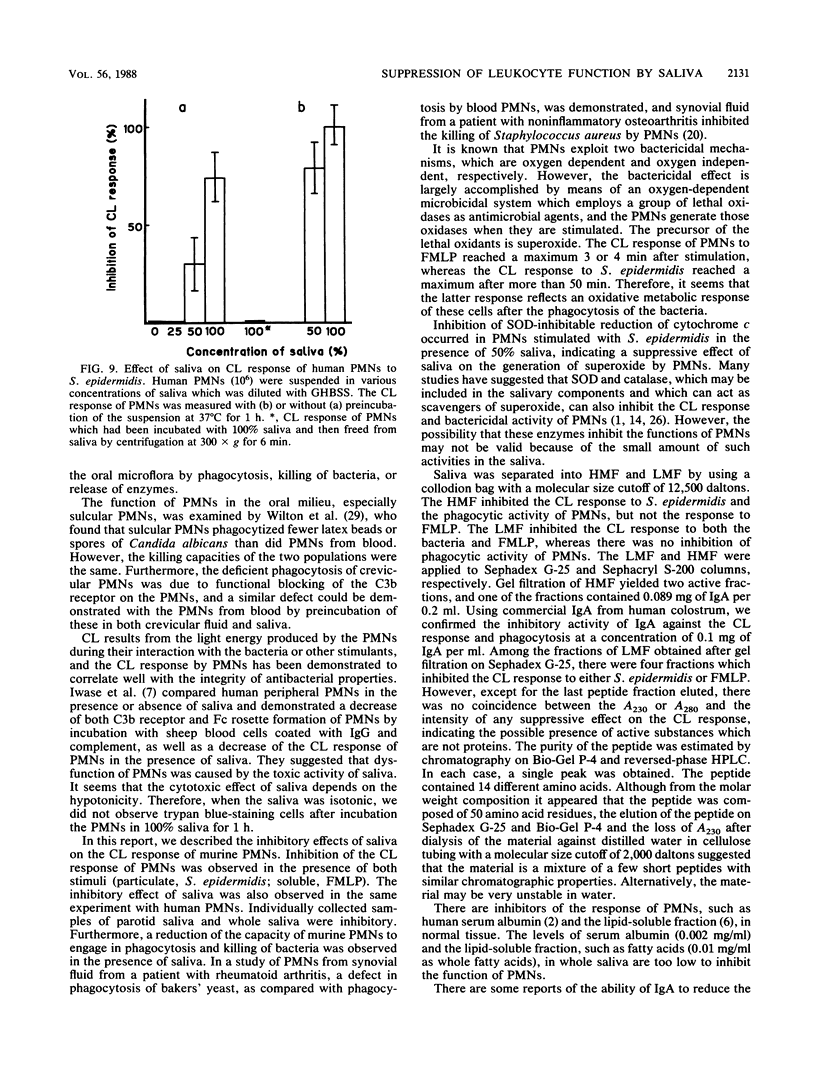
Saliva inhibited several functional properties of polymorphonuclear leukocytes (PMNs) from murine peritoneal exudate, namely, luminol-mediated chemiluminescence (CL) induced by either Staphylococcus epidermidis or formylmethionyl-leucyl-phenylalanine (FMLP), phagocytosis, and killing of bacteria in vitro. The concentration of saliva in the reaction mixture that caused a complete inhibition of the CL response of PMNs to both S. epidermidis and FMLP was 25%. However, there was no catalase or superoxide dismutase activity in saliva that could influence the CL response of PMNs. The production of superoxide by PMNs stimulated with S. epidermidis was assayed in the presence or absence of saliva by inhibition of the reduction of cytochrome c by superoxide dismutase. In the presence of 50% saliva, O2- generation by PMNs was only 7.3% of that observed in the absence of saliva. After gel filtration of salivary material through Sephadex G-25 or Sephacryl S-200, several fractions were obtained that inhibited the CL response of PMNs to either FMLP or S. epidermidis or to both. Two inhibitory fractions were analyzed. One contained immunoglobulin A, and the other contained a peptide which was composed of 14 different amino acids. The two fractions of high molecular weight included in the first protein peak of Sephacryl S-200 gel filtration were able to inhibit the CL response to S. epidermidis and to inhibit phagocytic activity, while fractions of low molecular weight (under 12,500 Mr) inhibited the CL response to FMLP and to S. epidermidis but did not inhibit phagocytic activity.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Briheim G., Stendahl O., Dahlgren C. Intra- and extracellular events in luminol-dependent chemiluminescence of polymorphonuclear leukocytes. Infect Immun. 1984 Jul;45(1):1–5. doi: 10.1128/iai.45.1.1-5.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanger M. W., Goldstine S. N., Shen L. The properties and role of receptors for IgA on human leukocytes. Ann N Y Acad Sci. 1983 Jun 30;409:552–563. doi: 10.1111/j.1749-6632.1983.tb26898.x. [DOI] [PubMed] [Google Scholar]
- Griffiss J. M., Bertram M. A. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J Infect Dis. 1977 Dec;136(6):733–739. doi: 10.1093/infdis/136.6.733. [DOI] [PubMed] [Google Scholar]
- Harrison J. E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976 Mar 10;251(5):1371–1374. [PubMed] [Google Scholar]
- Huang T. S., Hurd R. E., Chopra I. J., Stevens P., Solomon D. H., Young L. S. Inhibition of phagocytosis and chemiluminescence in human leukocytes by a lipid soluble factor in normal tissues. Infect Immun. 1984 Nov;46(2):544–551. doi: 10.1128/iai.46.2.544-551.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwase M., Kimura T., Nagae M., Ito S., Sakurada S., Nagumo M. [The functions of salivary neutrophils]. Nihon Shishubyo Gakkai Kaishi. 1985 Jun;27(2):388–393. doi: 10.2329/perio.27.388. [DOI] [PubMed] [Google Scholar]
- Kowashi Y., Jaccard F., Cimasoni G. Sulcular polymorphonuclear leucocytes and gingival exudate during experimental gingivitis in man. J Periodontal Res. 1980 Mar;15(2):151–158. doi: 10.1111/j.1600-0765.1980.tb00269.x. [DOI] [PubMed] [Google Scholar]
- Lantzman E., Michman J. Leukocyte counts in the saliva of adults before and after extraction of teeth. Oral Surg Oral Med Oral Pathol. 1970 Dec;30(6):766–773. doi: 10.1016/0030-4220(70)90335-x. [DOI] [PubMed] [Google Scholar]
- Markert M., Andrews P. C., Babior B. M. Measurement of O2- production by human neutrophils. The preparation and assay of NADPH oxidase-containing particles from human neutrophils. Methods Enzymol. 1984;105:358–365. doi: 10.1016/s0076-6879(84)05048-5. [DOI] [PubMed] [Google Scholar]
- Murray P. A., Patters M. R. Gingival crevice neutrophil function in periodontal lesions. J Periodontal Res. 1980 Sep;15(5):463–469. doi: 10.1111/j.1600-0765.1980.tb00304.x. [DOI] [PubMed] [Google Scholar]
- Musher D. M., Goree A., Baughn R. E., Birdsall H. H. Immunoglobulin A from bronchopulmonary secretions blocks bactericidal and opsonizing effects of antibody to nontypable Haemophilus influenzae. Infect Immun. 1984 Jul;45(1):36–40. doi: 10.1128/iai.45.1.36-40.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROVELSTAD G. H. SALIVARY CORPUSCLE ACTIVITY. J Am Dent Assoc. 1964 Mar;68:364–373. doi: 10.14219/jada.archive.1964.0085. [DOI] [PubMed] [Google Scholar]
- Repine J. E., Johansen K. S., Berger E. M. Hydroxyl radical scavengers produce similar decreases in the chemiluminescence responses and bactericidal activities of neutrophils. Infect Immun. 1984 Jan;43(1):435–437. doi: 10.1128/iai.43.1.435-437.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiött C. R., Löe H. The origin and variation in number of leukocytes in the human saliva. J Periodontal Res. 1970;5(1):36–41. doi: 10.1111/j.1600-0765.1970.tb01835.x. [DOI] [PubMed] [Google Scholar]
- Scully C. Phagocytic and killing activity of human blood, gingival crevicular, and salivary polymorphonuclear leukocytes for oral streptococci. J Dent Res. 1982 May;61(5):636–639. doi: 10.1177/00220345820610050301. [DOI] [PubMed] [Google Scholar]
- Simon G. L., Miller H. G., Borenstein D. G. Synovial fluid inhibits killing of Staphylococcus aureus by neutrophils. Infect Immun. 1983 Jun;40(3):1004–1010. doi: 10.1128/iai.40.3.1004-1010.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonis S. T., Mirando D., Stelos P., Lamster I. B. Capacity of human oral leucocytes to mediate antibody-dependent cell-mediated cytotoxicity. Arch Oral Biol. 1979;24(3):235–237. doi: 10.1016/0003-9969(79)90147-x. [DOI] [PubMed] [Google Scholar]
- Taichman N. S., Tsai C. C., Baehni P. C., Stoller N., McArthur W. P. Interaction of inflammatory cells and oral microorganisms. IV. In vitro release of lysosomal constituents from polymorphonuclear leukocytes exposed to supragingival and subgingival bacterial plaque. Infect Immun. 1977 Jun;16(3):1013–1023. doi: 10.1128/iai.16.3.1013-1023.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Epps D. E., Reed K., Williams R. C., Jr Suppression of human PMN bactericidal activity by human IgA paraproteins. Cell Immunol. 1978 Mar 15;36(2):363–376. doi: 10.1016/0008-8749(78)90280-0. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Williams R. C., Jr Suppression of leukocyte chemotaxis by human IgA myeloma components. J Exp Med. 1976 Nov 2;144(5):1227–1242. doi: 10.1084/jem.144.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb L. S., Keele B. B., Jr, Johnston R. B., Jr Inhibition of phagocytosis-associated chemiluminescence by superoxide dismutase. Infect Immun. 1974 Jun;9(6):1051–1056. doi: 10.1128/iai.9.6.1051-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann G., Zurier R. B., Spieler P. J., Goldstein I. M. Mechanisms of lysosomal enzyme release from leukocytes exposed to immune complexes and other particles. J Exp Med. 1971 Sep 1;134(3 Pt 2):149s–165s. [PubMed] [Google Scholar]
- Wilton J. M., Renggli H. H., Lehner T. A functional comparison of blood and gingival inflammatory polymorphonuclear leucocytes in man. Clin Exp Immunol. 1977 Jan;27(1):152–158. [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M., Renggli H. H., Lehner T. The role of Fc and C3b receptors in phagocytosis by inflammatory polymorphonuclear leucocytes in man. Immunology. 1977 Jun;32(6):955–961. [PMC free article] [PubMed] [Google Scholar]
- Wilton J. M. Suppression by IgA of IgG-mediated phagocytosis by human polymorphonuclear leucocytes. Clin Exp Immunol. 1978 Dec;34(3):423–428. [PMC free article] [PubMed] [Google Scholar]
- Woolweaver D. A., Koch G. G., Crawford J. J., Lundblad R. L. Relation of the orogranulocytic migratory rate to periodontal disease and blood leukocyte count: a clinical study. J Dent Res. 1972 Jul-Aug;51(4):929–939. doi: 10.1177/00220345720510043401. [DOI] [PubMed] [Google Scholar]



