Abstract
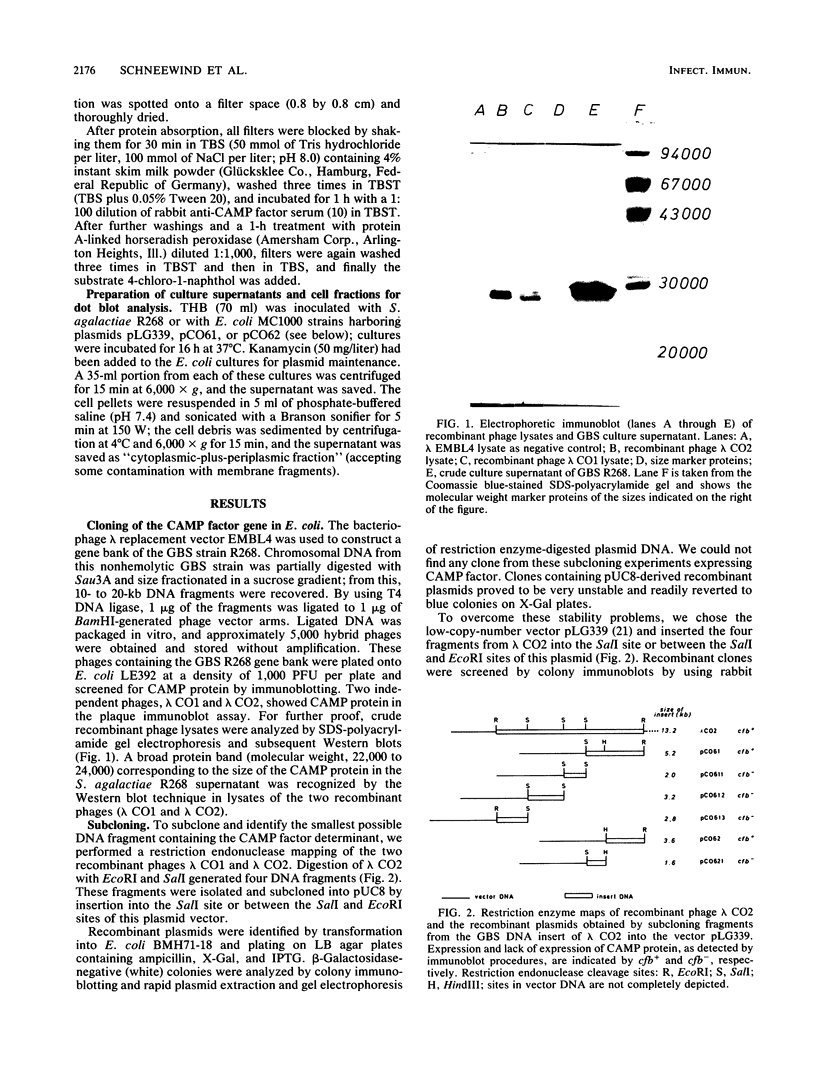
The genetic determinant of the CAMP factor from a strain of group B streptococcus (GBS; Streptococcus agalactiae) was cloned in Escherichia coli. Total cell DNA from the GBS strain R268 was used to construct a gene bank with bacteriophage lambda EMBL4 in the E. coli K-12 strain LE392. Recombinant phage plaques were detected by immunoblots by using a specific antiserum raised against purified CAMP factor. Two hybrid phages showing expression of CAMP factor were identified. Subcloning the CAMP gene (cfb) into the high-copy-number vector pUC8 resulted in highly unstable plasmids. Therefore, subcloning was performed with the low-copy-number vector pLG339 resulting in the stable recombinant plasmids pCO61 and pCO62 which lead to expression of CAMP protein first identified by colony immunoblotting. Western blot (immunoblot) analysis revealed a similar CAMP protein pattern in lambda EMBL4 recombinant phage lysates (molecular weight, 22,000 to 24,000) as compared to that obtained from a GBS culture supernatant (molecular weight, 22,000 to 26,000) but a different CAMP protein pattern (molecular weight, 20,000 to 23,000) from lysates of E. coli carrying pCO61 or pCO62. To study the excretion of the CAMP protein we performed a semi-quantitative dot blot analysis of proteins recovered from cell fractions and supernatants of the E. coli recombinant clones. In contrast to GBS R268, where the CAMP factor is readily excreted, the CAMP protein is not excreted in E. coli clones containing pCO61 and pCO62 but is found associated with the cell fractions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernheimer A. W., Linder R., Avigad L. S. Nature and mechanism of action of the CAMP protein of group B streptococci. Infect Immun. 1979 Mar;23(3):838–844. doi: 10.1128/iai.23.3.838-844.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Figura N., Guglielmetti P. Differentiation of motile and mesophilic Aeromonas strains into species by testing for a CAMP-like factor. J Clin Microbiol. 1987 Jul;25(7):1341–1342. doi: 10.1128/jcm.25.7.1341-1342.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jürgens D., Shalaby F. Y., Fehrenbach F. J. Purification and characterization of cAMP-factor from Streptococcus agalactiae by hydrophobic interaction chromatography and chromatofocusing. J Chromatogr. 1985 Dec 4;348(2):363–370. doi: 10.1016/s0021-9673(01)92474-4. [DOI] [PubMed] [Google Scholar]
- Jürgens D., Sterzik B., Fehrenbach F. J. Unspecific binding of group B streptococcal cocytolysin (CAMP factor) to immunoglobulins and its possible role in pathogenicity. J Exp Med. 1987 Mar 1;165(3):720–732. doi: 10.1084/jem.165.3.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karn J., Brenner S., Barnett L., Cesareni G. Novel bacteriophage lambda cloning vector. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5172–5176. doi: 10.1073/pnas.77.9.5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe M., Timmis K. N. Cloning and expression in Escherichia coli of the streptolysin O determinant from Streptococcus pyogenes: characterization of the cloned streptolysin O determinant and demonstration of the absence of substantial homology with determinants of other thiol-activated toxins. Infect Immun. 1984 Mar;43(3):804–810. doi: 10.1128/iai.43.3.804-810.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Messing J., Gronenborn B., Müller-Hill B., Hans Hopschneider P. Filamentous coliphage M13 as a cloning vehicle: insertion of a HindII fragment of the lac regulatory region in M13 replicative form in vitro. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3642–3646. doi: 10.1073/pnas.74.9.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka B., Smola J., Elischerová K. Routine test for in vitro differentiation of pathogenic and apathogenic Listeria monocytogenes strains. J Clin Microbiol. 1982 Mar;15(3):503–507. doi: 10.1128/jcm.15.3.503-507.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalka B., Smola J. Lethal effect of CAMP-factor and UBERIS-factor--a new finding about diffusible exosubstances of streptococcus agalactiae and Streptococcus uberis. Zentralbl Bakteriol A. 1981;249(2):190–194. [PubMed] [Google Scholar]
- So M., Crosa J. H., Falkow S. Polynucleotide sequence relationships among Ent plasmids and the relationship between Ent and other plasmids. J Bacteriol. 1975 Jan;121(1):234–238. doi: 10.1128/jb.121.1.234-238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]