Abstract
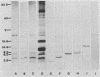
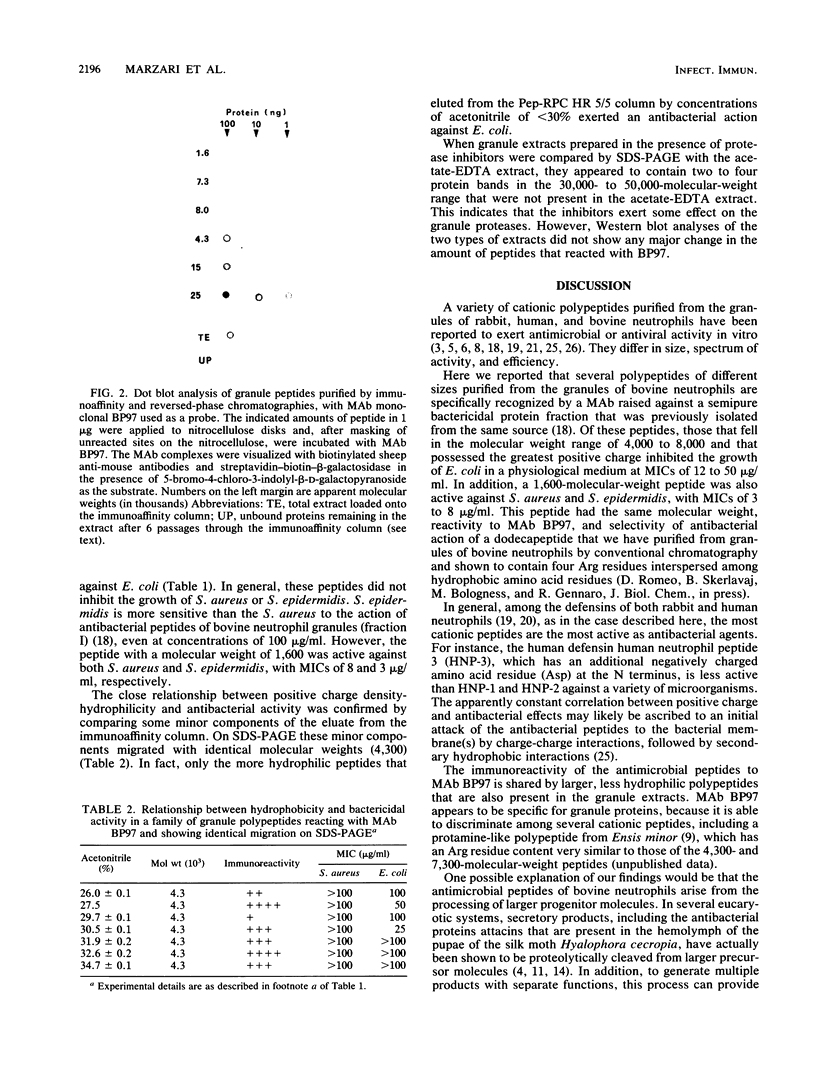
Monoclonal antibodies were raised against a bactericidal protein fraction that was purified from an extract of bovine neutrophil granules and that was previously shown (A. Savoini, R. Marzari, L. Dolzani, D. Serranò, G. Graziosi, R. Gennaro, and D. Romeo, Antimicrob. Agents Chemother. 26:405-407, 1984) to inhibit bacterial DNA synthesis. One of these antibodies, BP97, was covalently linked to Affi-Gel 10 and was used for immunoaffinity chromatography of granule extracts. Elution of the bound proteins, followed by reversed-phase high-performance liquid chromatography, generated several peptides whose molecular weights fell in the range of 1,600 to 64,000 and which reacted to BP97 but not to other mouse monoclonal or polyclonal antibodies. The reaction to BP97 appeared to be specific, inasmuch as a full panel of cationic oligo- or polypeptides was not recognized by this monoclonal antibody. Among the purified granule polypeptides, the more cationic ones (with molecular weights of 4,300 to 8,000) inhibited the growth of Escherichia coli at a MIC of 12 to 50 micrograms/ml. In addition, a 1,600-molecular-weight, highly cationic peptide also inhibited the growth of Staphylococcus aureus and Staphylococcus epidermidis at MICs of 3 to 8 micrograms/ml.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Daher K. A., Selsted M. E., Lehrer R. I. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986 Dec;60(3):1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglass J., Civelli O., Herbert E. Polyprotein gene expression: generation of diversity of neuroendocrine peptides. Annu Rev Biochem. 1984;53:665–715. doi: 10.1146/annurev.bi.53.070184.003313. [DOI] [PubMed] [Google Scholar]
- Elsbach P., Weiss J., Franson R. C., Beckerdite-Quagliata S., Schneider A., Harris L. Separation and purification of a potent bactericidal/permeability-increasing protein and a closely associated phospholipase A2 from rabbit polymorphonuclear leukocytes. Observations on their relationship. J Biol Chem. 1979 Nov 10;254(21):11000–11009. [PubMed] [Google Scholar]
- Ganz T., Selsted M. E., Szklarek D., Harwig S. S., Daher K., Bainton D. F., Lehrer R. I. Defensins. Natural peptide antibiotics of human neutrophils. J Clin Invest. 1985 Oct;76(4):1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro R., Dewald B., Horisberger U., Gubler H. U., Baggiolini M. A novel type of cytoplasmic granule in bovine neutrophils. J Cell Biol. 1983 Jun;96(6):1651–1661. doi: 10.1083/jcb.96.6.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro R., Dolzani L., Romeo D. Potency of bactericidal proteins purified from the large granules of bovine neutrophils. Infect Immun. 1983 May;40(2):684–690. doi: 10.1128/iai.40.2.684-690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giancotti V., Russo E., Gasparini M., Serrano D., Del Piero D., Thorne A. W., Cary P. D., Crane-Robinson C. Proteins from the sperm of the bivalve mollusc Ensis minor. Co-existence of histones and a protamine-like protein. Eur J Biochem. 1983 Nov 15;136(3):509–516. doi: 10.1111/j.1432-1033.1983.tb07770.x. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Julius D., Blair L., Brake A., Sprague G., Thorner J. Yeast alpha factor is processed from a larger precursor polypeptide: the essential role of a membrane-bound dipeptidyl aminopeptidase. Cell. 1983 Mar;32(3):839–852. doi: 10.1016/0092-8674(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Kockum K., Faye I., Hofsten P. V., Lee J. Y., Xanthopoulos K. G., Boman H. G. Insect immunity. Isolation and sequence of two cDNA clones corresponding to acidic and basic attacins from Hyalophora cecropia. EMBO J. 1984 Sep;3(9):2071–2075. doi: 10.1002/j.1460-2075.1984.tb02093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nishio C., Komura S., Kurahashi K. Peptide antibiotic subtilin is synthesized via precursor proteins. Biochem Biophys Res Commun. 1983 Oct 31;116(2):751–758. doi: 10.1016/0006-291x(83)90588-0. [DOI] [PubMed] [Google Scholar]
- Savoini A., Marzari R., Dolzani L., Serranò D., Graziosi G., Gennaro R., Romeo D. Wide-spectrum antibiotic activity of bovine granulocyte polypeptides. Antimicrob Agents Chemother. 1984 Sep;26(3):405–407. doi: 10.1128/aac.26.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsted M. E., Brown D. M., DeLange R. J., Harwig S. S., Lehrer R. I. Primary structures of six antimicrobial peptides of rabbit peritoneal neutrophils. J Biol Chem. 1985 Apr 25;260(8):4579–4584. [PubMed] [Google Scholar]
- Selsted M. E., Harwig S. S., Ganz T., Schilling J. W., Lehrer R. I. Primary structures of three human neutrophil defensins. J Clin Invest. 1985 Oct;76(4):1436–1439. doi: 10.1172/JCI112121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer W. M., Martin L. E., Spitznagel J. K. Cationic antimicrobial proteins isolated from human neutrophil granulocytes in the presence of diisopropyl fluorophosphate. Infect Immun. 1984 Jul;45(1):29–35. doi: 10.1128/iai.45.1.29-35.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiker S. A modification of the acetic acid-urea system for use in microslab polyacrylamide gel electrophoresis. Anal Biochem. 1980 Nov 1;108(2):263–265. doi: 10.1016/0003-2697(80)90579-5. [DOI] [PubMed] [Google Scholar]
- Spitznagel J. K., Pereira H. A., Martin L. E., Guzman G. S., Shafer W. M. A monoclonal antibody that inhibits the antimicrobial action of a 57 KD cationic protein of human polymorphonuclear leukocytes. J Immunol. 1987 Aug 15;139(4):1291–1296. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss J., Victor M., Elsbach P. Role of charge and hydrophobic interactions in the action of the bactericidal/permeability-increasing protein of neutrophils on gram-negative bacteria. J Clin Invest. 1983 Mar;71(3):540–549. doi: 10.1172/JCI110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerial A., Skerlavaj B., Gennaro R., Romeo D. Inactivation of herpes simplex virus by protein components of bovine neutrophil granules. Antiviral Res. 1987 Jul;7(6):341–352. doi: 10.1016/0166-3542(87)90016-7. [DOI] [PubMed] [Google Scholar]