Abstract
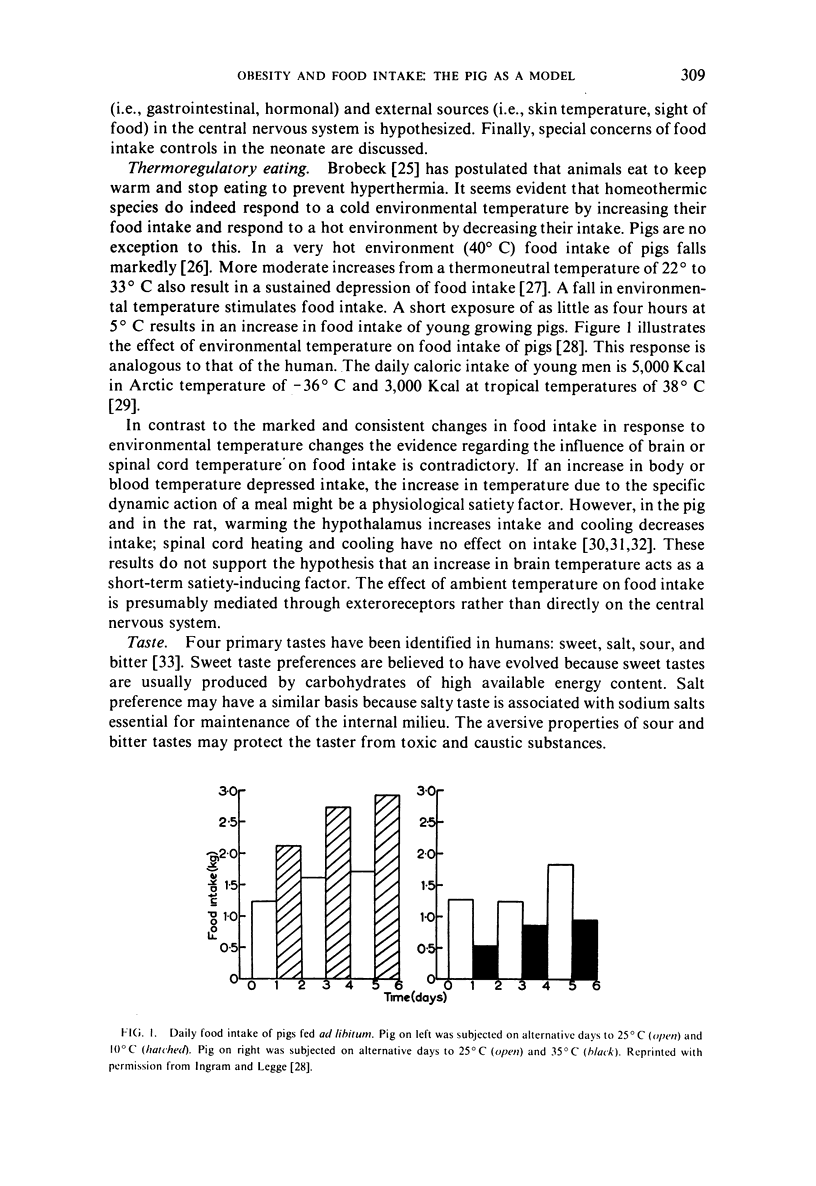
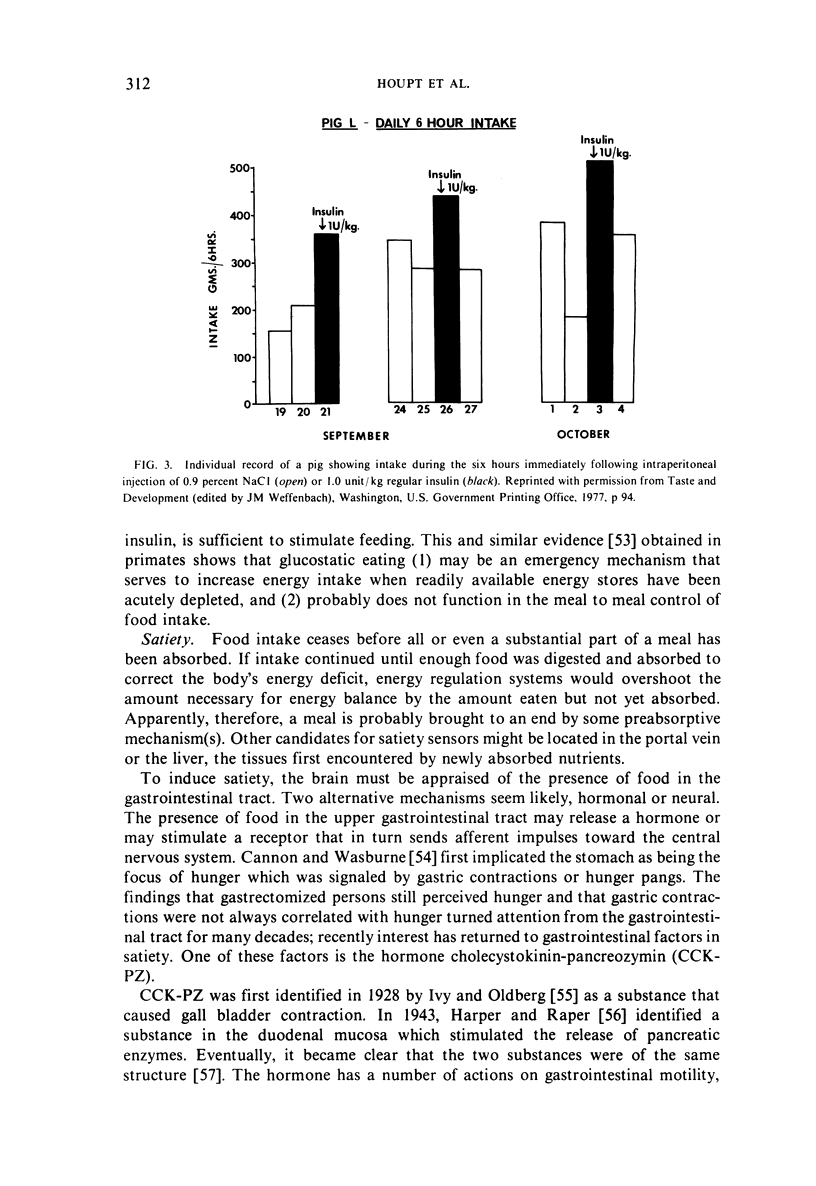
The use of the pig for studies of food intake and obesity is reviewed. Effects of ambient temperature and taste on food intake as well as satiety factors impicating both neural and hormonal mechanisms originating in the gastrointestinal tract are considered; the integration of information in the central nervous system for both internal and external sources is hypothesized. Special concerns of food intake controls in the neonate are discussed, including effects of neonate sweet preference on food intake, gastrointestinal satiety factors, and hypoglycemia as a stimulus for food ingestion.
For obesity studies, pigs offer several advantages, including their general physiological similarity to humans, similar fat cell size, and body fat distribution. Lipogenesis, lipolysis, and lipid mobilization are under intensive study in swine and the information obtained may have important application in studies of human obesity. The voluminous literature on metabolic differences between genetically lean versus obese populations of pigs suggests possibilities for application in humans. Greater characterization of differences and similarities between pigs and humans in important metabolic parameters related to regulation of food intake and obesity should facilitate better understanding and control of human obesity.
Full text
PDF


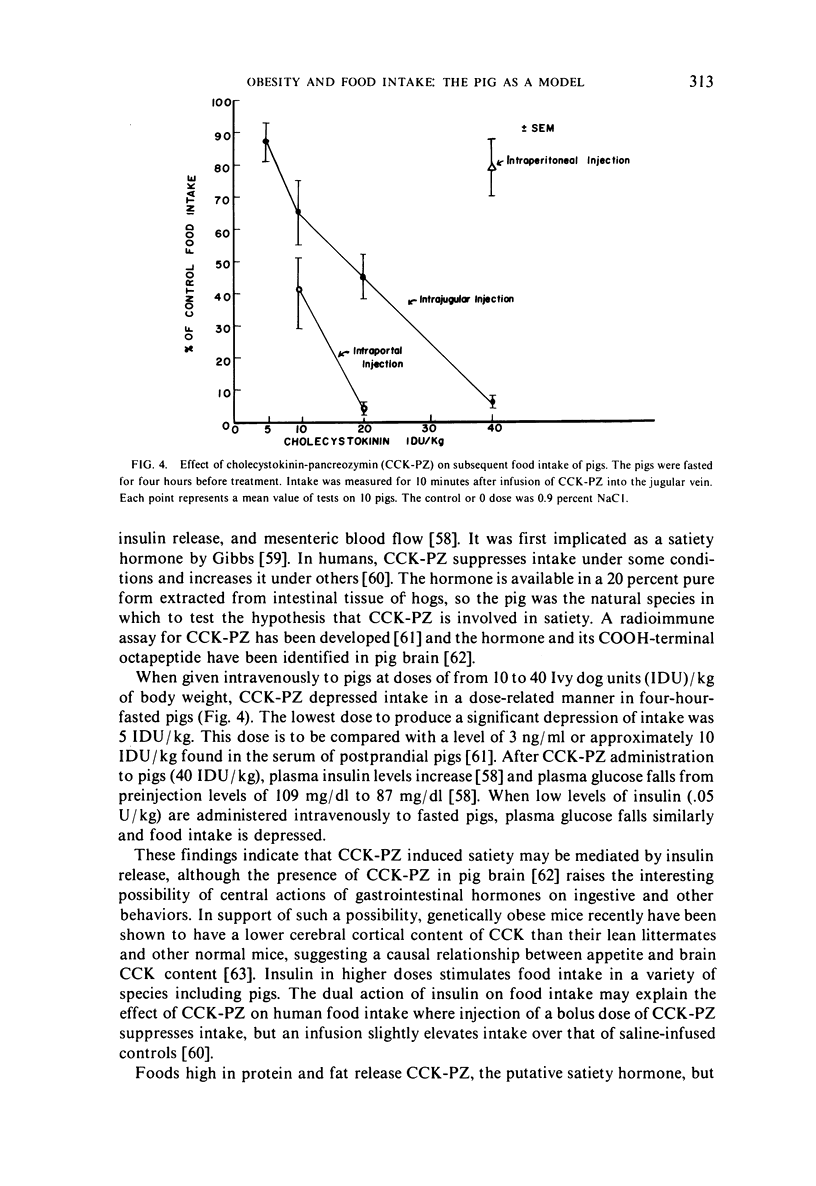









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANAND B. K., DUA S., SHOENBERG K. Hypothalamic control of food intake in cats and monkeys. J Physiol. 1955 Jan 28;127(1):143–152. doi: 10.1113/jphysiol.1955.sp005244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Althen T. G., Gerrits R. J. Metabolic clearance and secretion rates of porcine growth hormone in genetically lean and obese swine. Endocrinology. 1976 Aug;99(2):511–515. doi: 10.1210/endo-99-2-511. [DOI] [PubMed] [Google Scholar]
- Anderson D. B., Kauffman R. G. Cellular and enzymatic changes in porcine adipose tissue during growth. J Lipid Res. 1973 Mar;14(2):160–168. [PubMed] [Google Scholar]
- Anderson D. B., Kauffman R. G. Cellular and enzymatic changes in porcine adipose tissue during growth. J Lipid Res. 1973 Mar;14(2):160–168. [PubMed] [Google Scholar]
- BIELSCHOWSKY F., BIELSCHOWSKY M. The New Zealand strain of obese mice; their response to stilboestrol and to insulin. Aust J Exp Biol Med Sci. 1956 Jun;34(3):181–198. doi: 10.1038/icb.1956.22. [DOI] [PubMed] [Google Scholar]
- BROBECK J. R. Neural regulation of food intake. Ann N Y Acad Sci. 1955 Jul 15;63(1):44–55. doi: 10.1111/j.1749-6632.1955.tb36544.x. [DOI] [PubMed] [Google Scholar]
- Badger T. M., Tumbleson M. E., Hutcheson D. P. Protein-calorie malnutrition in young Sinclair(S-1) miniature swine. Growth. 1972 Sep;36(3):235–245. [PubMed] [Google Scholar]
- Baldwin B. A., Grovum W. L., Baile C. A., Brobeck J. R. Feeding following intraventricular injection of CA++, MG++ or pentobarbital in pigs. Pharmacol Biochem Behav. 1975 Sep-Oct;3(5):915–918. doi: 10.1016/0091-3057(75)90127-6. [DOI] [PubMed] [Google Scholar]
- Barlet J. P. The influence of porcine calcitonin given intragastrally on restraint-induced gastric ulcers in pigs. Horm Metab Res. 1974 Nov;6(6):517–521. doi: 10.1055/s-0028-1093801. [DOI] [PubMed] [Google Scholar]
- Barnes R. H., Moore A. U., Pond W. G. Behavioral abnormalities in young adult pigs caused by malnutrition in early life. J Nutr. 1970 Feb;100(2):149–155. doi: 10.1093/jn/100.2.149. [DOI] [PubMed] [Google Scholar]
- Berdanier C. D. The BHE strain to rat: an example of the role of inheritance in determining metabolic controls. Fed Proc. 1976 Sep;35(11):2295–2299. [PubMed] [Google Scholar]
- Book S. A., Bustad L. K. The fetal and neonatal pig in biomedical research. J Anim Sci. 1974 May;38(5):997–1002. doi: 10.2527/jas1974.385997x. [DOI] [PubMed] [Google Scholar]
- Bray G. A., Glennon J. A., Salans L. B., Horton E. S., Danforth E., Jr, Sims E. A. Spontaneous and experimental human obesity: effects of diet and adipose cell size on lipolysis and lipogenesis. Metabolism. 1977 Jul;26(7):739–747. doi: 10.1016/0026-0495(77)90061-0. [DOI] [PubMed] [Google Scholar]
- Bray G. A., York D. A. Genetically transmitted obesity in rodents. Physiol Rev. 1971 Jul;51(3):598–646. doi: 10.1152/physrev.1971.51.3.598. [DOI] [PubMed] [Google Scholar]
- Brobeck J. R., Tepperman J., Long C. N. Experimental Hypothalamic Hyperphagia in the Albino Rat. Yale J Biol Med. 1943 Jul;15(6):831–853. [PMC free article] [PubMed] [Google Scholar]
- Brobeck J. R., Tepperman J., Long C. N. Experimental Hypothalamic Hyperphagia in the Albino Rat. Yale J Biol Med. 1943 Jul;15(6):831–853. [PMC free article] [PubMed] [Google Scholar]
- Carlisle H. J., Ingram D. L. Effect on food intake in the pig of heating and cooling the spinal cord. Experientia. 1973 Oct 15;29(10):1241–1241. doi: 10.1007/BF01935095. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Effect of diazepam on performance of pigs in a progressive ratio schedule. Physiol Behav. 1976 Jul;17(1):161–163. doi: 10.1016/0031-9384(76)90286-9. [DOI] [PubMed] [Google Scholar]
- Davis M. A., Henry R., Leslie R. B. Comparative studies on porcine and human high density lipoproteins. Comp Biochem Physiol B. 1974 Apr 15;47(4):831–849. doi: 10.1016/0305-0491(74)90029-7. [DOI] [PubMed] [Google Scholar]
- Desor J. A., Greene L. S., Maller O. Preferences for sweet and salty in 9- to 15-year-old and adult humans. Science. 1975 Nov 14;190(4215):686–687. doi: 10.1126/science.1188365. [DOI] [PubMed] [Google Scholar]
- Dexter J. D., Tumbleson M. E., Hutcheson D. P., Middleton C. C. Sinclair(S-1) miniature swine as a model for the study of human alcoholism. Ann N Y Acad Sci. 1976;273:188–193. doi: 10.1111/j.1749-6632.1976.tb52881.x. [DOI] [PubMed] [Google Scholar]
- Enser M. Clearing-factor lipase in muscle and adipose tissue of pigs. Biochem J. 1973 Oct;136(2):381–385. doi: 10.1042/bj1360381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FILER L. J., Jr, CHURELLA H. RELATIONSHIP OF BODY COMPOSITION, CHEMICAL MATURATION, HOMEOSTASIS, AND DIET IN THE NEWBORN MAMMAL. Ann N Y Acad Sci. 1963 Sep 26;110:380–394. doi: 10.1111/j.1749-6632.1963.tb17103.x. [DOI] [PubMed] [Google Scholar]
- Faust I. M., Johnson P. R., Stern J. S., Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978 Sep;235(3):E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- Friend D. W. Self-selection of feeds and water by unbred gilts. J Anim Sci. 1973 Nov;37(5):1137–1141. doi: 10.2527/jas1973.3751137x. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Falasco J. D., McHugh P. R. Cholecystokinin-decreased food intake in rhesus monkeys. Am J Physiol. 1976 Jan;230(1):15–18. doi: 10.1152/ajplegacy.1976.230.1.15. [DOI] [PubMed] [Google Scholar]
- Gibbs J., Young R. C., Smith G. P. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol. 1973 Sep;84(3):488–495. doi: 10.1037/h0034870. [DOI] [PubMed] [Google Scholar]
- Go V. L., Ryan R. J., Summerskill W. H. Radioimmunoassay of porcine cholecystokinin-pancreozymin. J Lab Clin Med. 1971 Apr;77(4):684–689. [PubMed] [Google Scholar]
- Gurr M. I., Kirtland J., Phillip M., Robinson M. P. The consequences of early overnutrition for fat cell size and number: the pig as an experimental model for human obesity. Int J Obes. 1977;1(2):151–170. [PubMed] [Google Scholar]
- Harper A. A., Raper H. S. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol. 1943 Jun 30;102(1):115–125. doi: 10.1113/jphysiol.1943.sp004021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herberg L., Coleman D. L. Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism. 1977 Jan;26(1):59–99. doi: 10.1016/0026-0495(77)90128-7. [DOI] [PubMed] [Google Scholar]
- Hood R. L., Allen C. E. Cellularity of porcine adipose tissue: effects of growth and adiposity. J Lipid Res. 1977 May;18(3):275–284. [PubMed] [Google Scholar]
- Hood R. L., Allen C. E. Lipogenic enzyme activity in adipose tissue during the growth of swine with different propensities to fatten. J Nutr. 1973 Mar;103(3):353–362. doi: 10.1093/jn/103.3.353. [DOI] [PubMed] [Google Scholar]
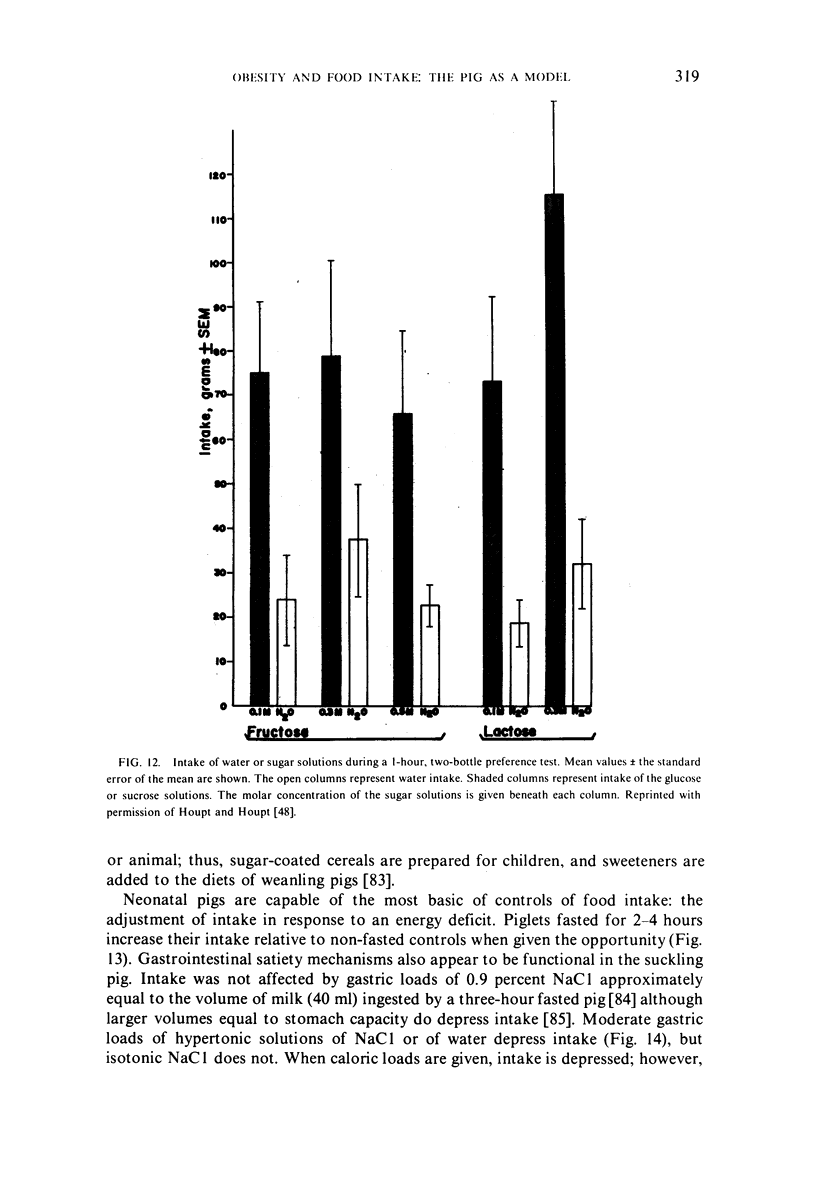
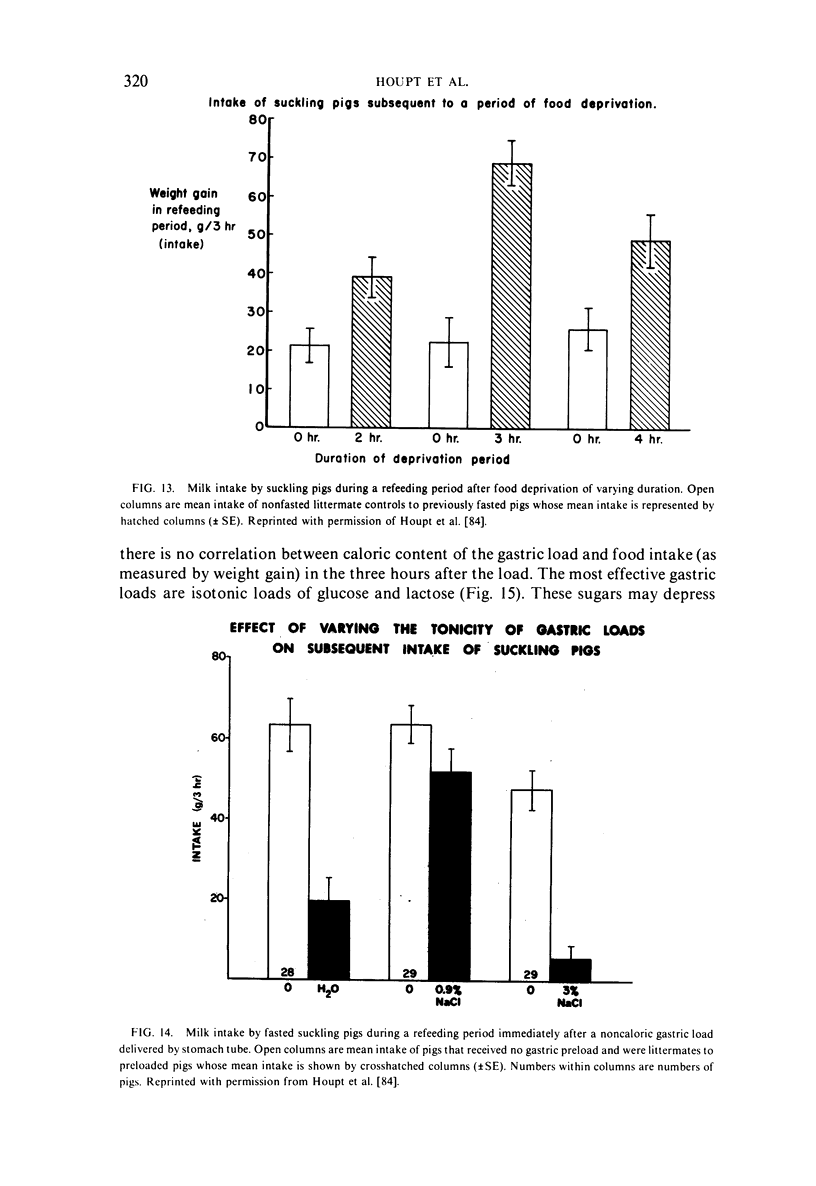
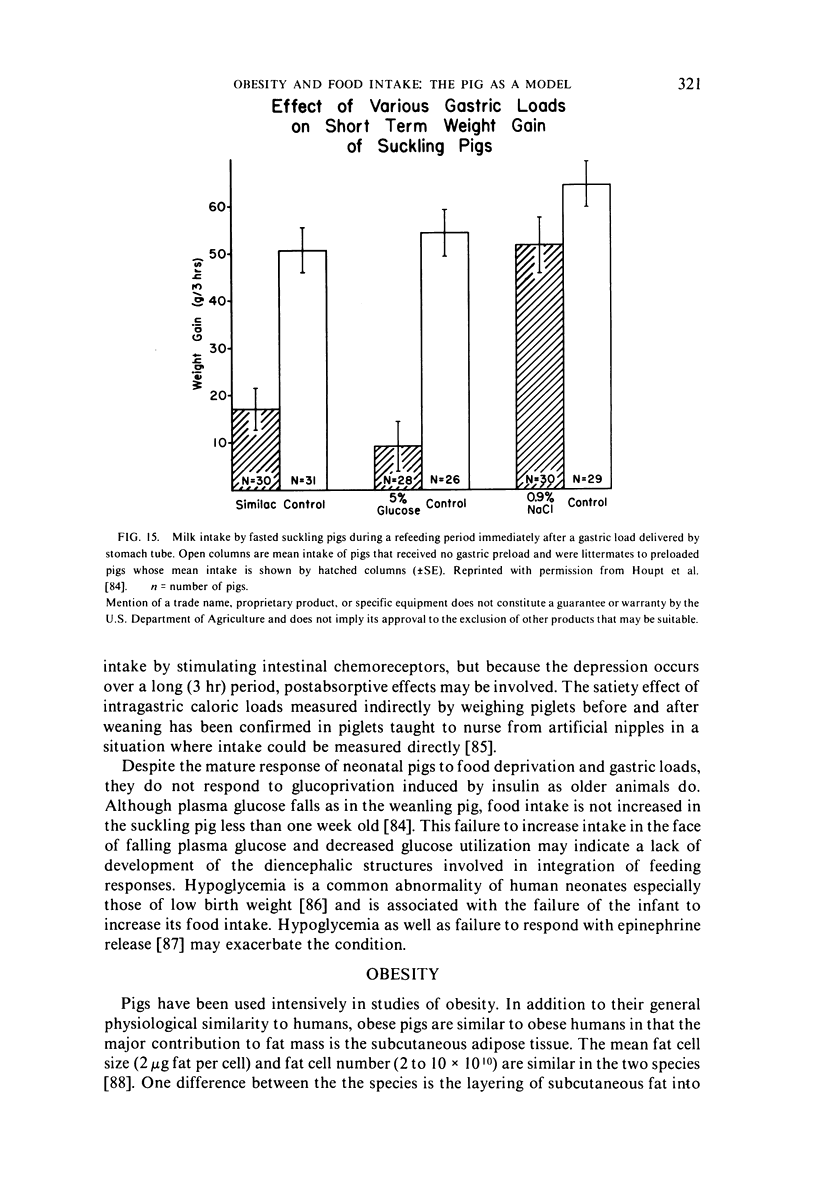
- Houpt K. A., Houpt T. R., Pond W. G. Food intake controls in the suckling pig: glucoprivation and gastrointestinal factors. Am J Physiol. 1977 May;232(5):E510–E514. doi: 10.1152/ajpendo.1977.232.5.E510. [DOI] [PubMed] [Google Scholar]
- Houpt T. R., Anika S. M., Wolff N. C. Satiety effects of cholecystokinin and caerulein in rabbits. Am J Physiol. 1978 Jul;235(1):R23–R28. doi: 10.1152/ajpregu.1978.235.1.R23. [DOI] [PubMed] [Google Scholar]
- Hunt C. E., Lindsey J. R., Walkley S. U. Animal models of diabetes and obesity, including the PBB/Ld mouse. Fed Proc. 1976 Apr;35(5):1206–1217. [PubMed] [Google Scholar]
- Hunt J. N., Pathak J. D. The osmotic effects of some simple molecules and ions on gastric emptying. J Physiol. 1960 Dec;154(2):254–269. doi: 10.1113/jphysiol.1960.sp006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram D. L. Effects of heating and cooling the hypothalamus on food intake in the pig. Brain Res. 1968 Dec;11(3):714–716. doi: 10.1016/0006-8993(68)90165-0. [DOI] [PubMed] [Google Scholar]
- Ingram D. L., Legge K. F. Effects of environmental temperature on food intake in growing pigs. Comp Biochem Physiol A Comp Physiol. 1974 Jul 1;48(3):573–581. doi: 10.1016/0300-9629(74)90740-3. [DOI] [PubMed] [Google Scholar]
- JANOWITZ H. D., GROSSMAN M. I. Some factors affecting the food intake of normal dogs and dogs with esophagostomy and gastric fistula. Am J Physiol. 1949 Oct;159(1):143–148. doi: 10.1152/ajplegacy.1949.159.1.143. [DOI] [PubMed] [Google Scholar]
- Jorpes J. E. The isolation and chemistry of secretin and cholecystokinin. Gastroenterology. 1968 Aug;55(2):157–164. [PubMed] [Google Scholar]
- Kare M. R., Pond W. C., Campbell J. Observations on the taste reactions in pigs. Anim Behav. 1965 Apr-Jul;13(2):265–269. doi: 10.1016/0003-3472(65)90045-x. [DOI] [PubMed] [Google Scholar]
- Kellogg T. F., Rogers R. W., Miller H. W. Differences in tissue fatty acids and cholesterol of swine from different genetic backgrounds. J Anim Sci. 1977 Jan;44(1):47–52. doi: 10.2527/jas1977.44147x. [DOI] [PubMed] [Google Scholar]
- Kennedy J. M., Baldwin B. A. Taste preferences in pigs for nutritive and non-nutritive sweet solutions. Anim Behav. 1972 Nov;20(4):706–718. doi: 10.1016/s0003-3472(72)80142-8. [DOI] [PubMed] [Google Scholar]
- Khalaf F. Hyperphagia and aphagia in swine with induced hypothalamic lesions. Res Vet Sci. 1969 Nov;10(6):514–517. [PubMed] [Google Scholar]
- Khalaf F., Robinson D. W. Aphagia and adipsia in pigs with induced hypothalamic lesions. Res Vet Sci. 1972 Jan;13(1):5–7. [PubMed] [Google Scholar]
- Khalaf F., Robinson D. W. Observations on the phagic response of the pig to infusions of dextrose and sodium pentobarbital into the ventromedial area of the brain. Res Vet Sci. 1972 Jan;13(1):1–4. [PubMed] [Google Scholar]
- Knittle J. L., Hirsch J. Effect of early nutrition on the development of rat epididymal fat pads: cellularity and metabolism. J Clin Invest. 1968 Sep;47(9):2091–2098. doi: 10.1172/JCI105894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEWIS L. A., PAGE I. H. Hereditary obesity: relation to serum lipoproteins and protein concentrations in swine. Circulation. 1956 Jul;14(1):55–59. doi: 10.1161/01.cir.14.1.55. [DOI] [PubMed] [Google Scholar]
- Laplace J. P. Circulation sanguine croisée chronique chez le porc. Technique chirurgicale, entretien, validité. J Physiol (Paris) 1972;64(2):165–172. [PubMed] [Google Scholar]
- Leveille G. A. In vivo fatty acid synthesis in adipose tissue and liver of meal-fed rats. Proc Soc Exp Biol Med. 1967 May;125(1):85–88. doi: 10.3181/00379727-125-32020. [DOI] [PubMed] [Google Scholar]
- MAYER J. Regulation of energy intake and the body weight: the glucostatic theory and the lipostatic hypothesis. Ann N Y Acad Sci. 1955 Jul 15;63(1):15–43. doi: 10.1111/j.1749-6632.1955.tb36543.x. [DOI] [PubMed] [Google Scholar]
- MOORE M. E., STUNKARD A., SROLE L. Obesity, social class, and mental illness. JAMA. 1962 Sep 15;181:962–966. doi: 10.1001/jama.1962.03050370030007. [DOI] [PubMed] [Google Scholar]
- Maller O., Turner R. E. Taste in acceptance of sugars by human infants. J Comp Physiol Psychol. 1973 Sep;84(3):496–501. doi: 10.1037/h0034906. [DOI] [PubMed] [Google Scholar]
- Martin R. J., Gobble J. L., Hartsock T. H., Graves H. B., Ziegler J. H. Characterization of an obese syndrome in the pig. Proc Soc Exp Biol Med. 1973 May;143(1):198–203. doi: 10.3181/00379727-143-37285. [DOI] [PubMed] [Google Scholar]
- Martin R. J., Herbein J. H. A comparison of the enzyme levels and the in vitro utilization of various substrates for lipogenesis in pair-fed lean and obese pigs. Proc Soc Exp Biol Med. 1976 Jan;151(1):231–235. doi: 10.3181/00379727-151-39180. [DOI] [PubMed] [Google Scholar]
- Masoro E. J. Fat metabolism in normal and abnormal states. Am J Clin Nutr. 1977 Aug;30(8):1311–1320. doi: 10.1093/ajcn/30.8.1311. [DOI] [PubMed] [Google Scholar]
- McHugh P. R., Gibbs J., Falasco J. D., Moran T., Smith G. P. Inhibitions of feeding examined in rhesus monkeys with hypothalamic disconnexions. Brain. 1975 Sep;98(3):441–454. doi: 10.1093/brain/98.3.441. [DOI] [PubMed] [Google Scholar]
- Merritt A. M., Brooks F. P. Basal and histamine-induced gastric acid and pepsin secretion in the conscious miniature pig. Gastroenterology. 1970 Jun;58(6):801–814. [PubMed] [Google Scholar]
- Mersmann H. J., Allen C. D., Steffen D. G., Brown L. G., Danielson D. M. Effect of age, weaning and diet on swine adipose tissue and liver lipogenesis. J Anim Sci. 1976 Jul;43(1):140–150. doi: 10.2527/jas1976.431140x. [DOI] [PubMed] [Google Scholar]
- Mersmann H. J., Brown L. J., Beuving R. D., Arakelian M. C. Lipolytic activity of swine adipocytes. Am J Physiol. 1976 May;230(5):1439–1443. doi: 10.1152/ajplegacy.1976.230.5.1439. [DOI] [PubMed] [Google Scholar]
- Mersmann H. J., Goodman J. R., Brown L. J. Development of swine adipose tissue: morphology and chemical composition. J Lipid Res. 1975 Jul;16(4):269–279. [PubMed] [Google Scholar]
- Mersmann H. J., Houk J. M., Phinney G., Underwood M. C., Brown L. J. Lipogenesis by in vitro liver and adipose tissue preparations from neonatal swine. Am J Physiol. 1973 May;224(5):1123–1129. doi: 10.1152/ajplegacy.1973.224.5.1123. [DOI] [PubMed] [Google Scholar]
- Mersmann H. J., Phinney G., Brown L. J. Factors influencing the lipolytic response of swine (Sus domesticus) adipose tissue: ontogeny. Biol Neonate. 1976;29(1-2):104–111. doi: 10.1159/000240854. [DOI] [PubMed] [Google Scholar]
- Mersmann H. J., Underwood M. C., Brown L. J., Houk J. M. Adipose tissue composition and lipogenic capacity in developing swine. Am J Physiol. 1973 May;224(5):1130–1135. doi: 10.1152/ajplegacy.1973.224.5.1130. [DOI] [PubMed] [Google Scholar]
- Muller J. E., Straus E., Yalow R. S. Cholecystokinin and its COOH-terminal octapeptide in the pig brain. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3035–3037. doi: 10.1073/pnas.74.7.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hea E. K., Leveille G. A. Influence of fasting and refeeding on lipogenesis and enzymatic activity of pig adipose tissue. J Nutr. 1969 Nov;99(3):345–352. doi: 10.1093/jn/99.3.345. [DOI] [PubMed] [Google Scholar]
- O'Hea E. K., Leveille G. A. Lipid metabolism in isolated adipose tissue of the domestic pig (Sus domesticus). Comp Biochem Physiol. 1968 Sep;26(3):1081–1089. doi: 10.1016/0010-406x(68)90028-5. [DOI] [PubMed] [Google Scholar]
- O'Hea E. K., Leveille G. A. Significance of adipose tissue and liver as sites of fatty acid synthesis in the pig and the efficiency of utilization of various substrates for lipogenesis. J Nutr. 1969 Nov;99(3):338–344. doi: 10.1093/jn/99.3.338. [DOI] [PubMed] [Google Scholar]
- POND W. G., BARNES R. H., BRADFIELD R. B., KWONG E., KROOK L. EFFECT OF DIETARY ENERGY INTAKE ON PROTEIN DEFICIENCY SYMPTOMS AND BODY COMPOSITION OF BABY PIGS FED EQUALIZED BUT SUBOPTIMAL AMOUNTS OF PROTEIN. J Nutr. 1965 Jan;85:57–66. doi: 10.1093/jn/85.1.57. [DOI] [PubMed] [Google Scholar]
- Pekas J. C. Permanent physiological fistula of the pancreas and other digestive glands. J Appl Physiol. 1965 Sep;20(5):1082–1084. doi: 10.1152/jappl.1965.20.5.1082. [DOI] [PubMed] [Google Scholar]
- Pond W. G., Snyder W., Snook J. T., Walker E. F., Jr, McNeill D. A., Stillings B. R. Relative utilization of casein, fish protein concentrate and isolated soybean protein for growth and pancreatic enzyme regeneration of the protein-calorie malnourished baby pig. J Nutr. 1971 Sep;101(9):1193–1200. doi: 10.1093/jn/101.9.1193. [DOI] [PubMed] [Google Scholar]
- Ratcliffe H. L., Luginbühl H., Schnarr W. R., Chacko K. Coronary arteriosclerosis in swine: evidence of a relation to behavior. J Comp Physiol Psychol. 1969 Jul;68(3):385–392. doi: 10.1037/h0027520. [DOI] [PubMed] [Google Scholar]
- Robinson D. W. Food intake regulation in pigs. II. The relationship between dietary protein concentration, body composition, cellular development and the efficiency of protein and energy utilization. Br Vet J. 1974 Sep-Oct;130(5):424–433. doi: 10.1016/s0007-1935(17)35784-6. [DOI] [PubMed] [Google Scholar]
- Robinson D. W. Food intake regulation in pigs. III. Voluntary food selection between protein-free and protein-rich diets. Br Vet J. 1974 Nov-Dec;130(6):522–527. doi: 10.1016/s0007-1935(17)35737-8. [DOI] [PubMed] [Google Scholar]
- Rothschild M. F., Cahpman A. B. Factors influencing serum cholesterol levels in swine. J Hered. 1976 Jan-Feb;67(1):47–48. doi: 10.1093/oxfordjournals.jhered.a108663. [DOI] [PubMed] [Google Scholar]
- Schneider D. L., Sarett H. P. Nutritional studies on hysterectomy-obtained SPF baby pigs fed infant formula products. J Nutr. 1966 Jun;89(2):158–164. doi: 10.1093/jn/89.2.158. [DOI] [PubMed] [Google Scholar]
- Schneider D. L., Sarett H. P. Use of the hysterectomy-obtained SPF pig for nutritional studies of the neonate. J Nutr. 1966 May;89(1):43–48. doi: 10.1093/jn/89.1.43. [DOI] [PubMed] [Google Scholar]
- Sheng H. P., Kamonsakpithak S., Naiborhu A., Chuntananukoon S., Huggins R. A. Measured and calculated fat during growth in the pig and beagle. Growth. 1977 Jun;41(2):139–146. [PubMed] [Google Scholar]
- Sims E. A. Experimental obesity, dietary-induced thermogenesis, and their clinical implications. Clin Endocrinol Metab. 1976 Jul;5(2):377–395. doi: 10.1016/s0300-595x(76)80027-8. [DOI] [PubMed] [Google Scholar]
- Sizonenko P. C., Paunier L., Vallotton B., Cuendet G. S., Zahnd G., Marliss E. B. Response to 2-deoxy-D-glucose and to glucagon in "ketotic hypoglycemia" of childhood: evidence for epinephrine deficiency and altered alanine availability. Pediatr Res. 1973 Dec;7(12):983–993. doi: 10.1203/00006450-197312000-00007. [DOI] [PubMed] [Google Scholar]
- Smith G. P., Epstein A. N. Increased feeding in response to decreased glucose utilization in the rat and monkey. Am J Physiol. 1969 Oct;217(4):1083–1087. doi: 10.1152/ajplegacy.1969.217.4.1083. [DOI] [PubMed] [Google Scholar]
- Smith G. P., Gibbs J., Strohmayer A. J., Stokes P. E. Threshold doses of 2-deoxy-D-glucose for hyperglycemia and feeding in rats and monkeys. Am J Physiol. 1972 Jan;222(1):77–81. doi: 10.1152/ajplegacy.1972.222.1.77. [DOI] [PubMed] [Google Scholar]
- Spector N. H., Brobeck J. R., Hamilton C. L. Feeding and core temperature in albino rats: changes induced by preoptic heating and cooling. Science. 1968 Jul 19;161(3838):286–288. doi: 10.1126/science.161.3838.286. [DOI] [PubMed] [Google Scholar]
- Spiegel T. A. Caloric regulation of food intake in man. J Comp Physiol Psychol. 1973 Jul;84(1):24–37. doi: 10.1037/h0035006. [DOI] [PubMed] [Google Scholar]
- Stadaas J., Aune S., Haffner J. F. Effects of proximal gastric vagotomy on intragastric pressure and adaptation in pigs. Scand J Gastroenterol. 1974;9(5):479–485. [PubMed] [Google Scholar]
- Steele N. C., Frobish L. T., Keeney M. Lipogenesis and cellularity of adipose tissue from genetically lean and obese swine. J Anim Sci. 1974 Oct;39(4):712–719. doi: 10.2527/jas1974.394712x. [DOI] [PubMed] [Google Scholar]
- Steele N. C., Frobish L. T. Selected lipogenic enzyme activities of swine adipose tissue as influenced by genetic phenotype, age, feeding frequency and dietary energy source. Growth. 1976 Dec;40(4):369–378. [PubMed] [Google Scholar]
- Steffen D. G., Chai E. Y., Brown L. J., Mersmann H. J. Effects of diet on swine glyceride lipid metabolism. J Nutr. 1978 Jun;108(6):911–918. doi: 10.1093/jn/108.6.911. [DOI] [PubMed] [Google Scholar]
- Stephens D. B. Effects of gastric loading on the sucking response and voluntary milk intake in neonatal piglets. J Comp Physiol Psychol. 1975 Feb;88(2):796–805. doi: 10.1037/h0076391. [DOI] [PubMed] [Google Scholar]
- Straus E., Yalow R. S. Cholecystokinin in the brains of obese and nonobese mice. Science. 1979 Jan 5;203(4375):68–69. doi: 10.1126/science.758680. [DOI] [PubMed] [Google Scholar]
- Sturdevant R. A., Goetz H. Cholecystokinin both stimulates and inhibits human food intake. Nature. 1976 Jun 24;261(5562):713–715. doi: 10.1038/261713a0. [DOI] [PubMed] [Google Scholar]
- Trygstad O., Foss I., Vold E., Standal N. Suppressed lipolysis in genetically fat pigs. FEBS Lett. 1972 Oct 1;26(1):311–314. doi: 10.1016/0014-5793(72)80600-8. [DOI] [PubMed] [Google Scholar]
- Walike B. C., Smith O. A. Regulation of food intake during intermittent and continuous cross circulation in monkeys (Macaca mulatta). J Comp Physiol Psychol. 1972 Sep;80(3):372–381. doi: 10.1037/h0032982. [DOI] [PubMed] [Google Scholar]
- Wangsness P. J., Martin R. J., Gahagan J. H. Insulin and growth hormone in lean and obese pigs. Am J Physiol. 1977 Aug;233(2):E104–E108. doi: 10.1152/ajpendo.1977.233.2.E104. [DOI] [PubMed] [Google Scholar]


