Abstract
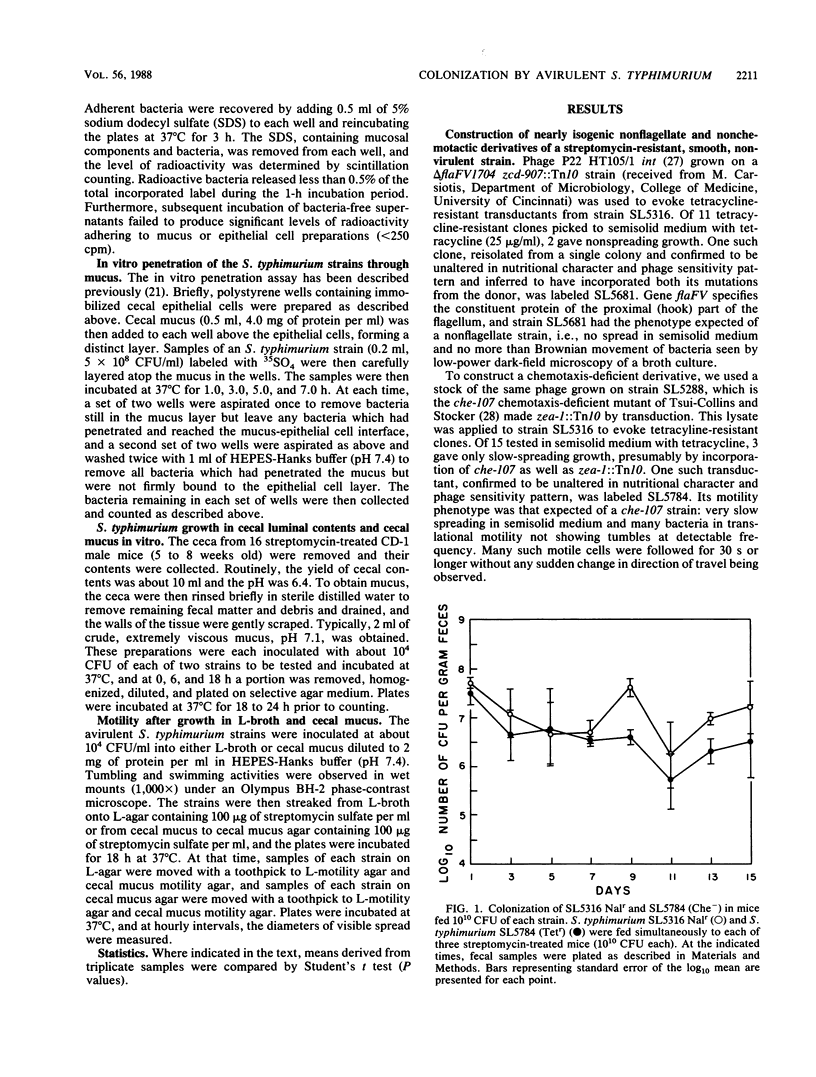
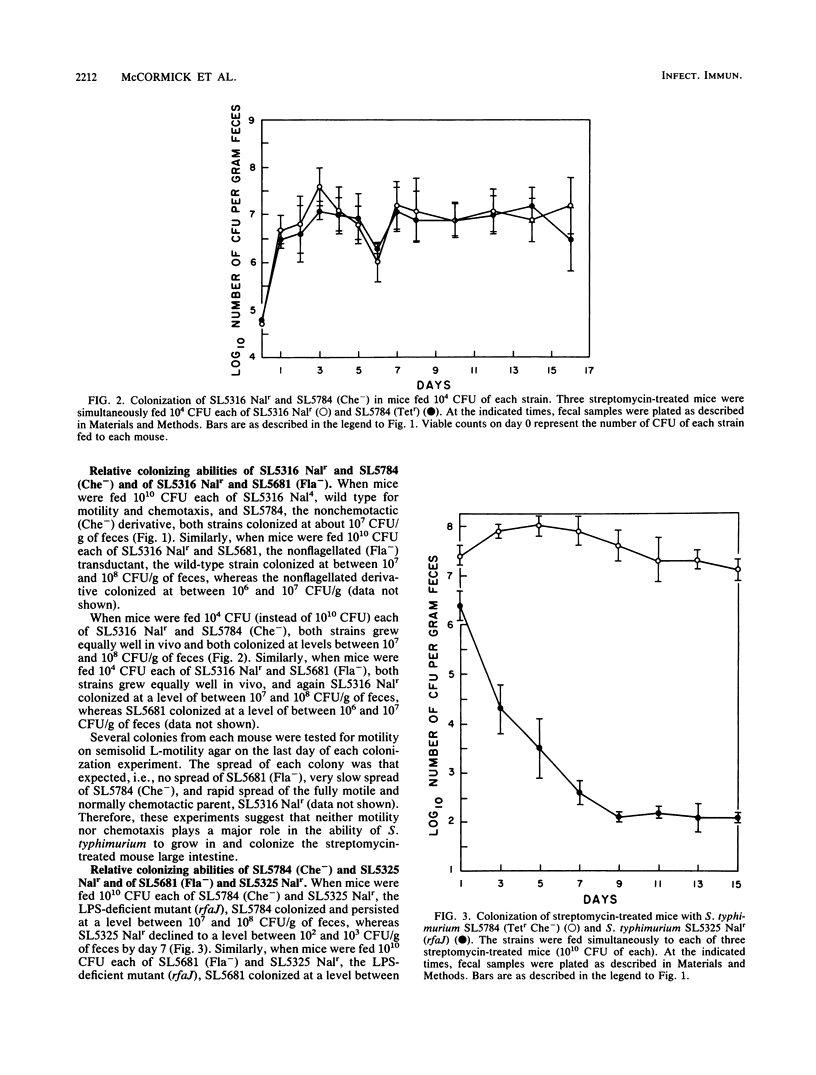
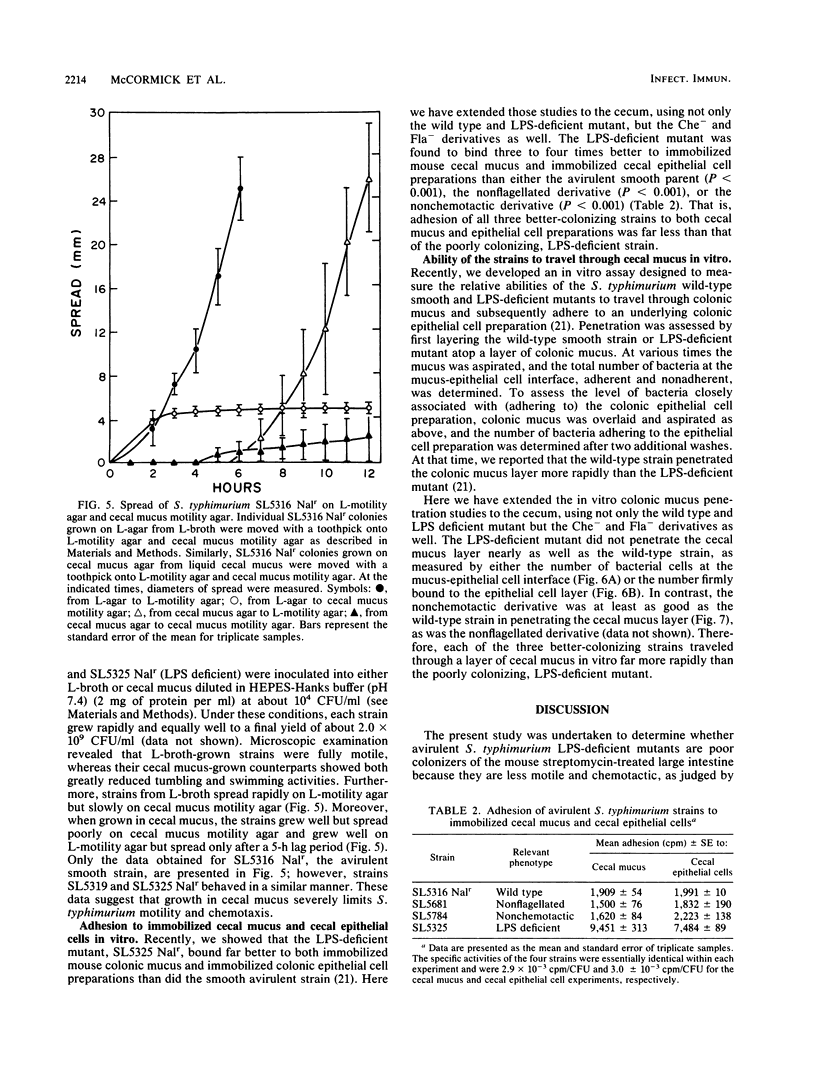
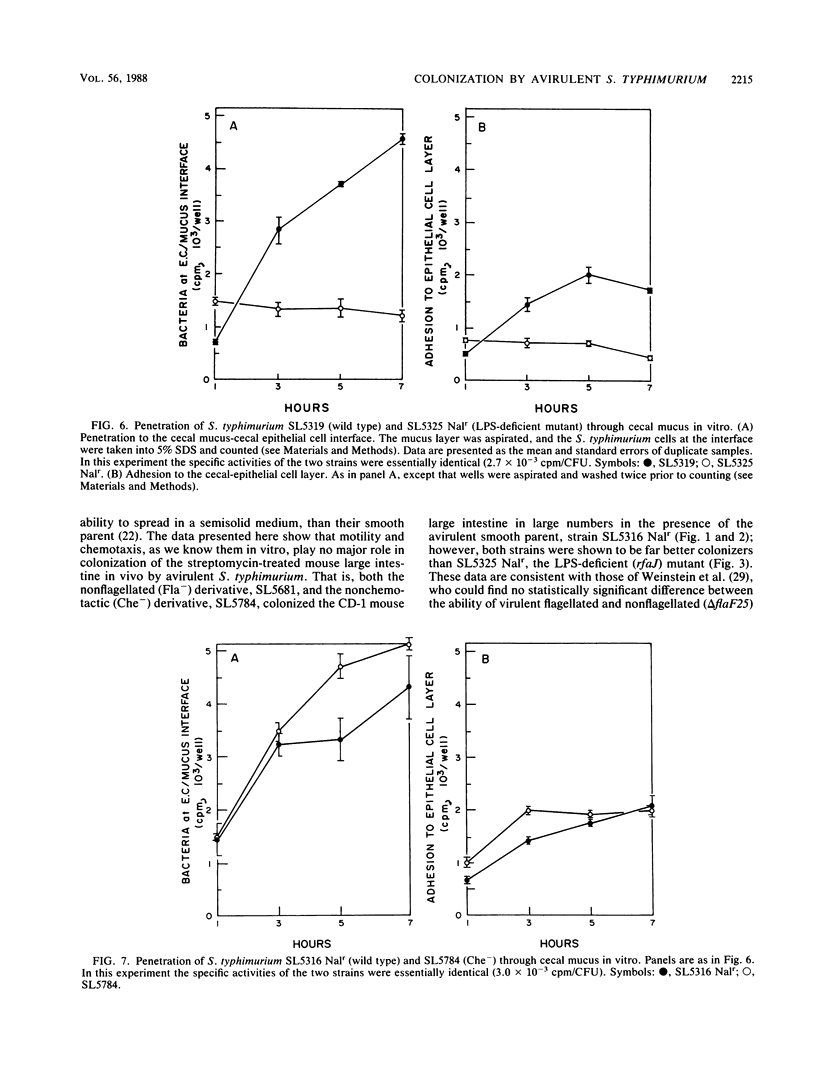
Previously, it had been shown that an avirulent strain of Salmonella typhimurium, SL5316, with wild-type lipopolysaccharide (LPS) was a far better colonizer of the streptomycin-treated CD-1 mouse large intestine, was far more motile, did not bind to mouse intestinal mucus nearly as well as, but penetrated through a layer of intestinal mucus in vitro far better than an almost isogenic LPS-deficient transductant, SL5325. In the present investigation, a nonflagellated transductant, SL5681, and a nonchemotactic transductant, SL5784, were isolated from SL5316 and tested for relative colonizing ability versus SL5316 (smooth) and SL5325 (rough) in streptomycin-treated mice. In addition, the Salmonella strains were tested for their ability to grow together in cecal intestinal mucus and in cecal luminal contents, for their tumbling and swimming activities after growth in cecal mucus, and for their ability to adhere to and travel through cecal mucus in vitro. The data show that the nonflagellated and nonchemotactic derivatives colonized large intestine nearly as well as their parent and were far better colonizers than the LPS-deficient mutant, that all the strains grew equally well in cecal mucus but did not grow in cecal luminal contents, and that cecal mucus-grown strains lost tumbling and swimming activities. Furthermore, the LPS-deficient strain adhered to cecal mucus far better but penetrated mucus far worse than did the nonflagellated transductant, the nonchemotactic transductant, and the parent. Thus, motility and chemotaxis do not appear to play a major role in the ability of the avirulent S. typhimurium strains to colonize the mouse large intestine, colonization may require growth in cecal mucus but does not depend on growth in cecal luminal contents, growth in cecal mucus inhibits S. typhimurium motility, and increased adhesion of the LPS-deficient mutant to cecal mucus and its poor ability to penetrate cecal mucus may play a role in its poor intestine-colonizing ability.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOHNHOFF M., MILLER C. P., MARTIN W. R. RESISTANCE OF THE MOUSE'S INTESTINAL TRACT TO EXPERIMENTAL SALMONELLA INFECTION. II. FACTORS RESPONSIBLE FOR ITS LOSS FOLLOWING STREPTOMYCIN TREATMENT. J Exp Med. 1964 Nov 1;120:817–828. doi: 10.1084/jem.120.5.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Arruda J. C., Williams T. J., Laux D. C. Adhesion of a human fecal Escherichia coli strain to mouse colonic mucus. Infect Immun. 1985 Apr;48(1):139–145. doi: 10.1128/iai.48.1.139-145.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A. L., Stocker B. A. Salmonella typhimurium mutants generally defective in chemotaxis. J Bacteriol. 1976 Dec;128(3):754–765. doi: 10.1128/jb.128.3.754-765.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duguid J. P., Anderson E. S., Alfredsson G. A., Barker R., Old D. C. A new biotyping scheme for Salmonella typhimurium and its phylogenetic significance. J Med Microbiol. 1975 Feb;8(1):149–166. doi: 10.1099/00222615-8-1-149. [DOI] [PubMed] [Google Scholar]
- Freter R., Allweiss B., O'Brien P. C., Halstead S. A., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vitro studies. Infect Immun. 1981 Oct;34(1):241–249. doi: 10.1128/iai.34.1.241-249.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Botney M., Cleven D., Aranki A. Mechanisms that control bacterial populations in continuous-flow culture models of mouse large intestinal flora. Infect Immun. 1983 Feb;39(2):676–685. doi: 10.1128/iai.39.2.676-685.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., Brickner H., Fekete J., Vickerman M. M., Carey K. E. Survival and implantation of Escherichia coli in the intestinal tract. Infect Immun. 1983 Feb;39(2):686–703. doi: 10.1128/iai.39.2.686-703.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freter R., O'Brien P. C., Macsai M. S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981 Oct;34(1):234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hoskins L. C., Zamcheck N. Bacterial degradation of gastrointestinal mucins. I. Comparison of mucus constituents in the stools of germ-free and conventional rats. Gastroenterology. 1968 Feb;54(2):210–217. [PubMed] [Google Scholar]
- Komeda Y., Icho T., Iino T. Effects of galU mutation on flagellar formation in Escherichia coli. J Bacteriol. 1977 Feb;129(2):908–915. doi: 10.1128/jb.129.2.908-915.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux D. C., McSweegan E. F., Williams T. J., Wadolkowski E. A., Cohen P. S. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab). Infect Immun. 1986 Apr;52(1):18–25. doi: 10.1128/iai.52.1.18-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevola J. J., Laux D. C., Cohen P. S. In vivo colonization of the mouse large intestine and in vitro penetration of intestinal mucus by an avirulent smooth strain of Salmonella typhimurium and its lipopolysaccharide-deficient mutant. Infect Immun. 1987 Dec;55(12):2884–2890. doi: 10.1128/iai.55.12.2884-2890.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevola J. J., Stocker B. A., Laux D. C., Cohen P. S. Colonization of the mouse intestine by an avirulent Salmonella typhimurium strain and its lipopolysaccharide-defective mutants. Infect Immun. 1985 Oct;50(1):152–159. doi: 10.1128/iai.50.1.152-159.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que J. U., Casey S. W., Hentges D. J. Factors responsible for increased susceptibility of mice to intestinal colonization after treatment with streptomycin. Infect Immun. 1986 Jul;53(1):116–123. doi: 10.1128/iai.53.1.116-123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revel H. R. Restriction of nonglucosylated T-even bacteriophage: properties of permissive mutants of Escherichia coli B and K12. Virology. 1967 Apr;31(4):688–701. doi: 10.1016/0042-6822(67)90197-3. [DOI] [PubMed] [Google Scholar]
- Rubulis A., Rubert M., Faloon W. W. Cholesterol lowering, fecal bile acid, and sterol changes during neomycin and colchicine. Am J Clin Nutr. 1970 Sep;23(9):1251–1259. doi: 10.1093/ajcn/23.9.1251. [DOI] [PubMed] [Google Scholar]
- Schmieger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119(1):75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- Weinstein D. L., Carsiotis M., Lissner C. R., O'Brien A. D. Flagella help Salmonella typhimurium survive within murine macrophages. Infect Immun. 1984 Dec;46(3):819–825. doi: 10.1128/iai.46.3.819-825.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]



