Abstract
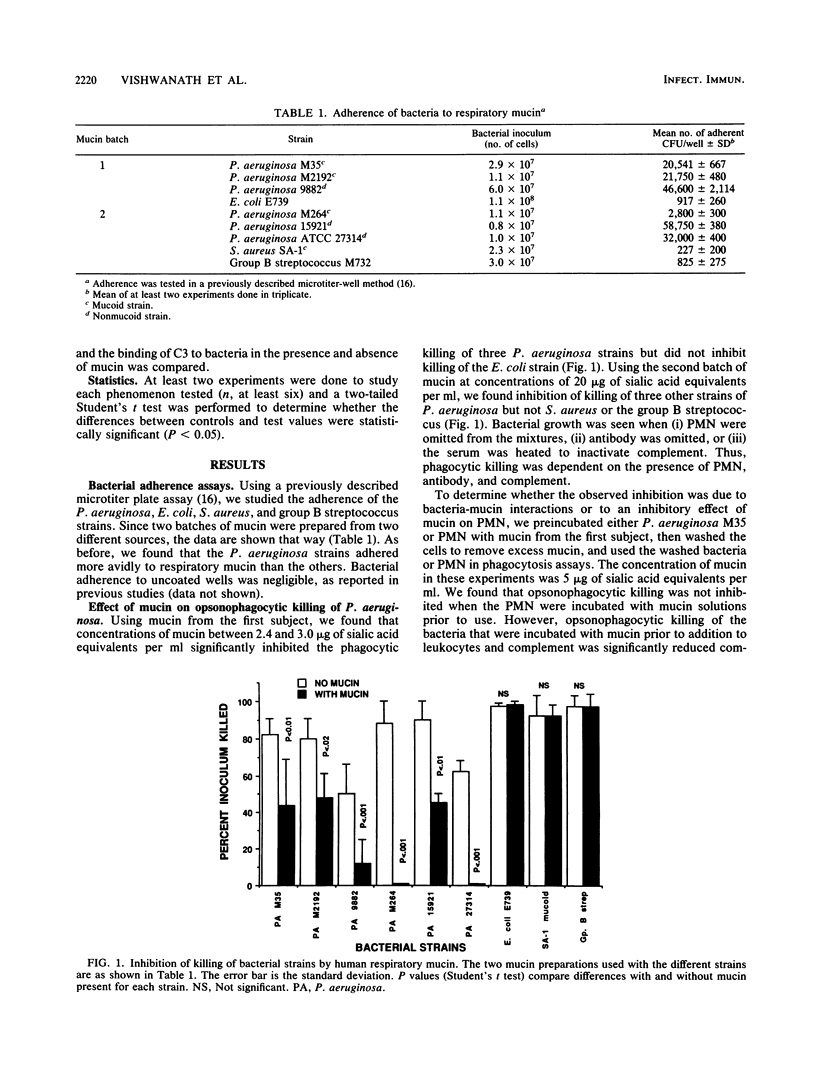
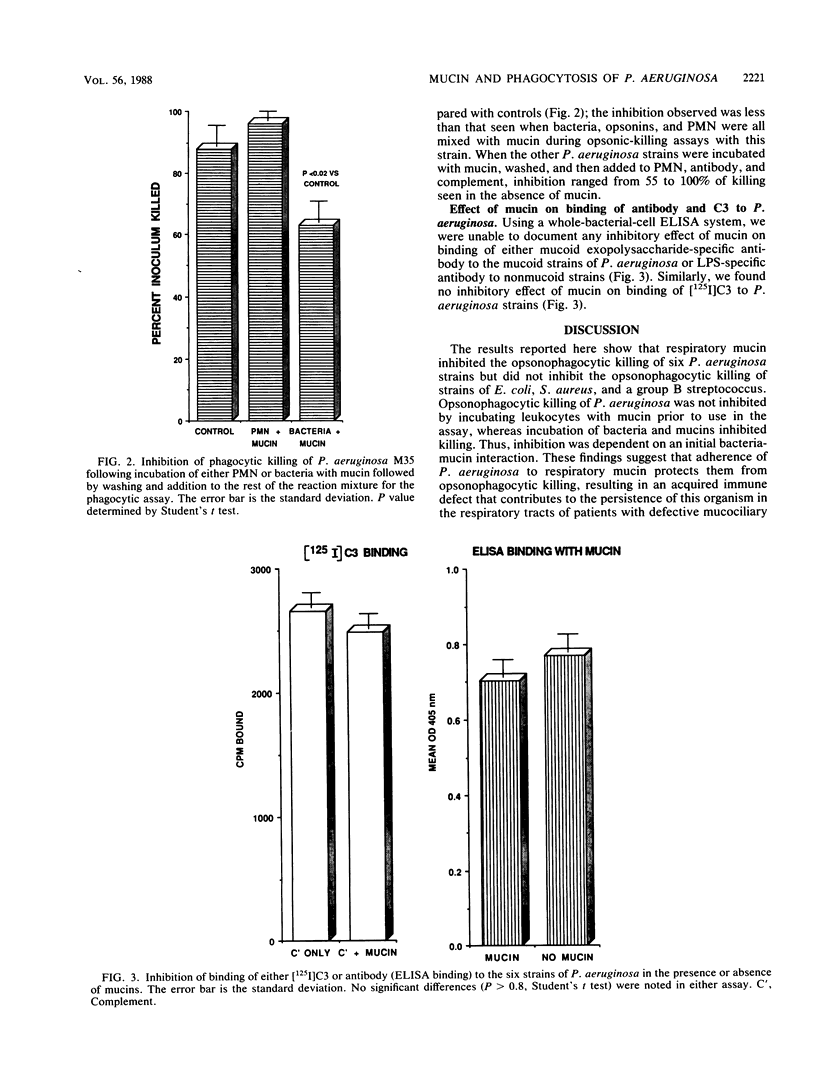
Pseudomonas aeruginosa is a frequent respiratory tract colonizer in diseases in which mucociliary clearance is defective. The most striking of these is cystic fibrosis. The reasons for this organism's ability to colonize the respiratory tract and to persist there are not fully understood. Earlier studies showed that P. aeruginosa adheres preferentially to tracheobronchial mucin when compared with enterobacteria. We reasoned that if adherence to respiratory mucin protected P. aeruginosa from opsonophagocytic killing, then the ability of this organism to chronically colonize the respiratory tract could be partially explained. Using an opsonophagocytic killing assay with human polymorphonuclear leukocytes, we found that respiratory mucin protected six strains of P. aeruginosa from opsonophagocytic killing but did not protect poorly adhering strains of Escherichia coli, Staphylococcus aureus, or group B streptococci. Incubating P. aeruginosa with the mucin prior to addition to the opsonic assay inhibited phagocytic killing, whereas incubation of polymorphonuclear leukocytes with mucin did not, suggesting that inhibition was not due to an effect of mucin on leukocytes per se but was a consequence of bacterial adherence to mucin. Further studies indicated no decrease in the binding of either antibody or complement component C3 to the bacterial surface in the presence of mucin. This suggests that phagocytic inhibition may be due to a defect in uptake or destruction of mucin-coated bacteria by the leukocytes. Thus, the adherence of P. aeruginosa to respiratory mucin potentially contributes to its persistence in the respiratory tract by interfering with host immune responses.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames P., DesJardins D., Pier G. B. Opsonophagocytic killing activity of rabbit antibody to Pseudomonas aeruginosa mucoid exopolysaccharide. Infect Immun. 1985 Aug;49(2):281–285. doi: 10.1128/iai.49.2.281-285.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedrossian C. W., Greenberg S. D., Singer D. B., Hansen J. J., Rosenberg H. S. The lung in cystic fibrosis. A quantitative study including prevalence of pathologic findings among different age groups. Hum Pathol. 1976 Mar;7(2):195–204. doi: 10.1016/s0046-8177(76)80023-8. [DOI] [PubMed] [Google Scholar]
- Cohen P. S., Rossoll R., Cabelli V. J., Yang S. L., Laux D. C. Relationship between the mouse colonizing ability of a human fecal Escherichia coli strain and its ability to bind a specific mouse colonic mucous gel protein. Infect Immun. 1983 Apr;40(1):62–69. doi: 10.1128/iai.40.1.62-69.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. S., Zollinger W., Mandrell R., Gemski P., Sadoff J. Evaluation of immunotherapeutic approaches for the potential treatment of infections caused by K1-positive Escherichia coli. J Infect Dis. 1983 Jan;147(1):68–76. doi: 10.1093/infdis/147.1.68. [DOI] [PubMed] [Google Scholar]
- Döring G., Obernesser H. J., Botzenhart K., Flehmig B., Høiby N., Hofmann A. Proteases of Pseudomonas aeruginosa in patients with cystic fibrosis. J Infect Dis. 1983 Apr;147(4):744–750. doi: 10.1093/infdis/147.4.744. [DOI] [PubMed] [Google Scholar]
- Edwards M. S., Kasper D. L., Jennings H. J., Baker C. J., Nicholson-Weller A. Capsular sialic acid prevents activation of the alternative complement pathway by type III, group B streptococci. J Immunol. 1982 Mar;128(3):1278–1283. [PubMed] [Google Scholar]
- Fick R. B., Jr, Baltimore R. S., Squier S. U., Reynolds H. Y. IgG proteolytic activity of Pseudomonas aeruginosa in cystic fibrosis. J Infect Dis. 1985 Apr;151(4):589–598. doi: 10.1093/infdis/151.4.589. [DOI] [PubMed] [Google Scholar]
- Kuriyama S. M., Silverblatt F. J. Effect of Tamm-Horsfall urinary glycoprotein on phagocytosis and killing of type I-fimbriated Escherichia coli. Infect Immun. 1986 Jan;51(1):193–198. doi: 10.1128/iai.51.1.193-198.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. C., Michon F., Perez N. E., Hopkins C. A., Pier G. B. Chemical characterization and immunogenicity of capsular polysaccharide isolated from mucoid Staphylococcus aureus. Infect Immun. 1987 Sep;55(9):2191–2197. doi: 10.1128/iai.55.9.2191-2197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Desjardins D., Aguilar T., Barnard M., Speert D. P. Polysaccharide surface antigens expressed by nonmucoid isolates of Pseudomonas aeruginosa from cystic fibrosis patients. J Clin Microbiol. 1986 Aug;24(2):189–196. doi: 10.1128/jcm.24.2.189-196.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pier G. B., Matthews W. J., Jr, Eardley D. D. Immunochemical characterization of the mucoid exopolysaccharide of Pseudomonas aeruginosa. J Infect Dis. 1983 Mar;147(3):494–503. doi: 10.1093/infdis/147.3.494. [DOI] [PubMed] [Google Scholar]
- Suter S., Schaad U. B., Roux L., Nydegger U. E., Waldvogel F. A. Granulocyte neutral proteases and Pseudomonas elastase as possible causes of airway damage in patients with cystic fibrosis. J Infect Dis. 1984 Apr;149(4):523–531. doi: 10.1093/infdis/149.4.523. [DOI] [PubMed] [Google Scholar]
- Vishwanath S., Ramphal R. Adherence of Pseudomonas aeruginosa to human tracheobronchial mucin. Infect Immun. 1984 Jul;45(1):197–202. doi: 10.1128/iai.45.1.197-202.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitnack E., Beachey E. H. Inhibition of complement-mediated opsonization and phagocytosis of Streptococcus pyogenes by D fragments of fibrinogen and fibrin bound to cell surface M protein. J Exp Med. 1985 Dec 1;162(6):1983–1997. doi: 10.1084/jem.162.6.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



