Abstract
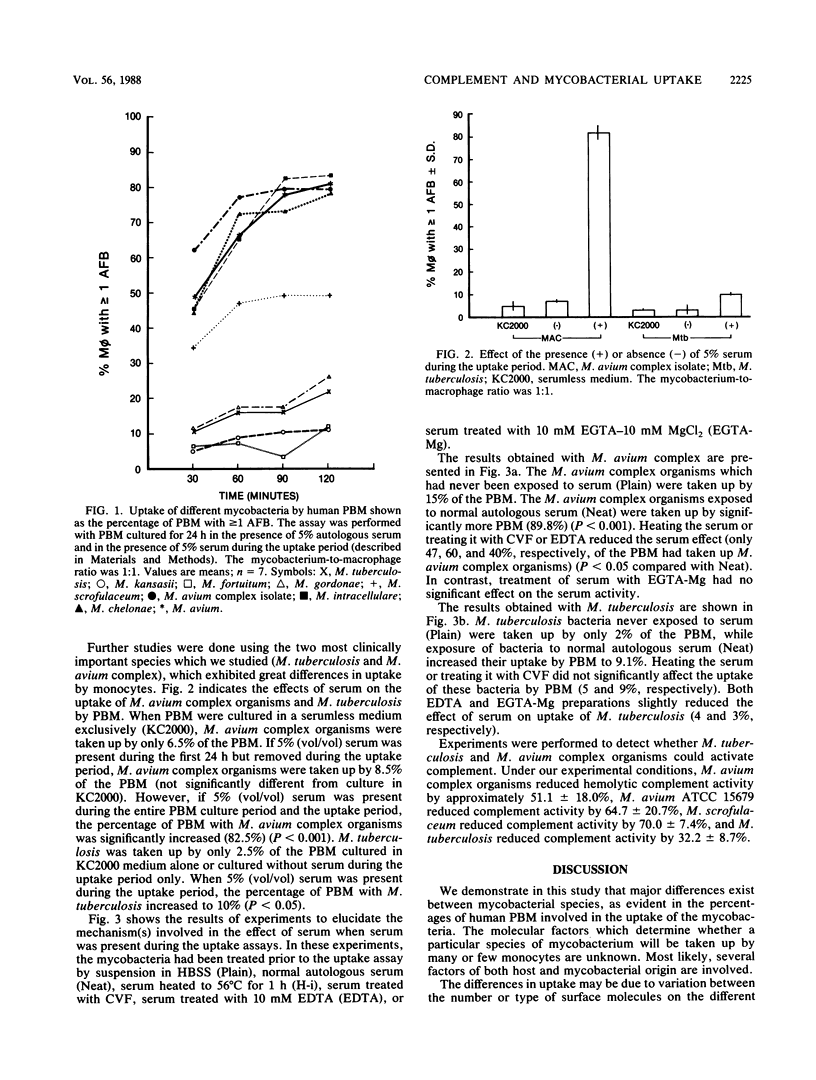
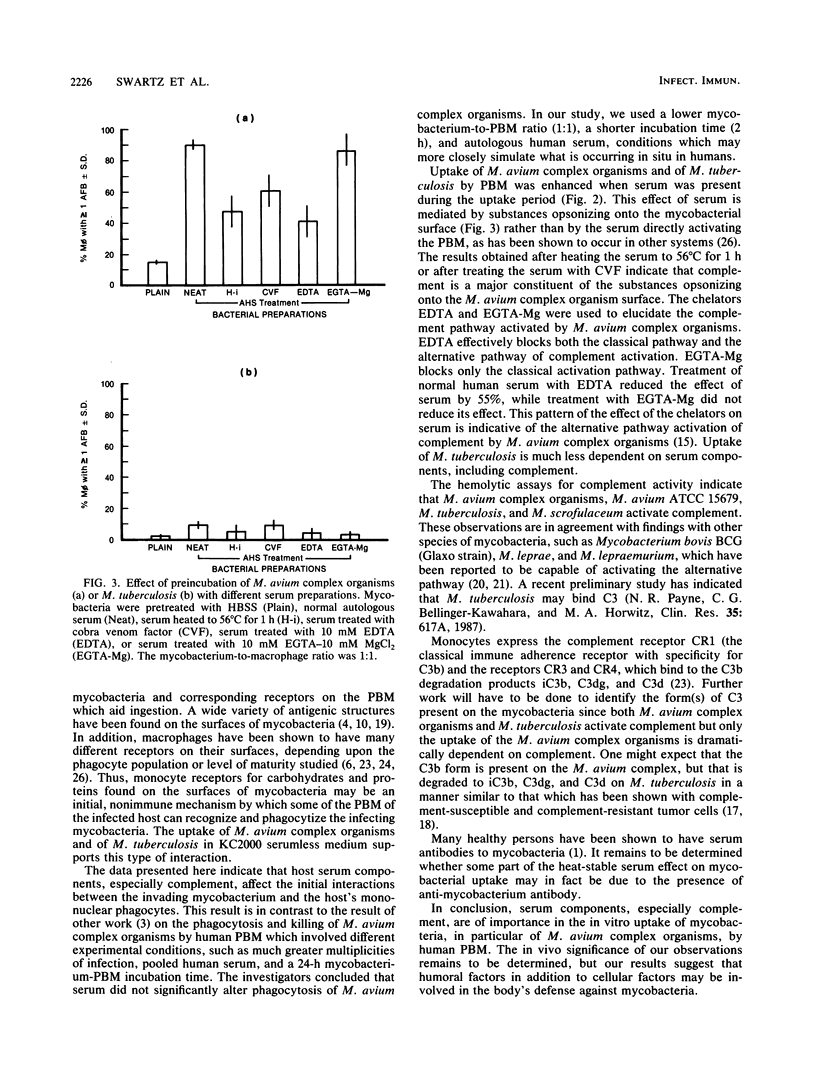
We investigated the influence of serum factors on the uptake of various species of mycobacteria by human peripheral blood monocytes (PBM). On the basis of the percentage of PBM involved during in vitro uptake, the mycobacteria were of two distinct groups. The mycobacteria of one group, which consisted of Mycobacterium avium complex and M. chelonae, were taken up by many PBM; the other group, consisting of M. tuberculosis, M. kansasii, M. fortuitum, and M. gordonae, were taken up by fewer PBM. M. scrofulaceum was intermediate to these two groups on the basis of its uptake by PBM. Serum depleted of complement by heating or treatment with cobra venom factor significantly reduced the extent of PBM involvement with M. avium complex, indicating that complement is an important serum component mediating the uptake of M. avium complex organisms. Preincubation of mycobacteria with serum containing 10 mM EGTA [ethylene glycol-bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid] and 10 mM MgCl2 resulted in uptake by a high percentage of PBM, while preincubation in heated serum or serum containing 10 mM EDTA resulted in a significantly reduced percentage of PBM involved in uptake of M. avium complex organisms, indicating that these organisms are activators of the alternative pathway of complement. Incubation of M. avium complex organisms in human serum consumed 51% of the hemolytic complement activity. Parallel experiments indicated that serum had a lesser effect on the uptake of M. tuberculosis. Thus, serum is important in in vitro M. avium complex uptake by PBM; complement has a major role in the effect of serum, but this role is less important with M. tuberculosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardana E. J., Jr, McClatchy J. K., Farr R. S., Minden P. Universal occurrence of antibodies to tubercle bacilli in sera from non-tuberculous and tuberculous individuals. Clin Exp Immunol. 1973 Jan;13(1):65–77. [PMC free article] [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Phagocytosis and intracellular killing of Mycobacterium avium complex by human and murine macrophages. Braz J Med Biol Res. 1987;20(2):191–201. [PubMed] [Google Scholar]
- Bermudez L. E., Young L. S. Tumor necrosis factor, alone or in combination with IL-2, but not IFN-gamma, is associated with macrophage killing of Mycobacterium avium complex. J Immunol. 1988 May 1;140(9):3006–3013. [PubMed] [Google Scholar]
- Bullock W. E., Wright S. D. Role of the adherence-promoting receptors, CR3, LFA-1, and p150,95, in binding of Histoplasma capsulatum by human macrophages. J Exp Med. 1987 Jan 1;165(1):195–210. doi: 10.1084/jem.165.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaparas S. D. The immunology of mycobacterial infections. Crit Rev Microbiol. 1982;9(2):139–197. doi: 10.3109/10408418209104488. [DOI] [PubMed] [Google Scholar]
- Collins F. M. The immunology of tuberculosis. Am Rev Respir Dis. 1982 Mar;125(3 Pt 2):42–49. doi: 10.1164/arrd.1982.125.3P2.42. [DOI] [PubMed] [Google Scholar]
- Crowle A. J. Studies of antituberculosis chemotherapy with an in vitro model of human tuberculosis. Semin Respir Infect. 1986 Dec;1(4):262–264. [PubMed] [Google Scholar]
- Edwards D., Kirkpatrick C. H. The immunology of mycobacterial diseases. Am Rev Respir Dis. 1986 Nov;134(5):1062–1071. doi: 10.1164/arrd.1986.134.5.1062. [DOI] [PubMed] [Google Scholar]
- Fine D. P., Marney S. R., Jr, Colley D. G., Sergent J. S., Des Prez R. M. C3 shunt activation in human serum chelated with EGTA. J Immunol. 1972 Oct;109(4):807–809. [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Mason U. G., 3rd, Farhi D. C., Iseman M. D. Disseminated infection with Mycobacterium avium-intracellulare. A report of 13 cases and a review of the literature. Medicine (Baltimore) 1985 Jan;64(1):36–48. doi: 10.1097/00005792-198501000-00003. [DOI] [PubMed] [Google Scholar]
- Kitz D. J., Johnson C. R., Kobayashi G. S., Medoff G., Little J. R. Growth inhibition of Cryptococcus neoformans by cloned cultured murine macrophages. Cell Immunol. 1984 Oct 15;88(2):489–500. doi: 10.1016/0008-8749(84)90180-1. [DOI] [PubMed] [Google Scholar]
- Panneerselvam M., Bredehorst R., Vogel C. W. Immobilized doxorubicin increases the complement susceptibility of human melanoma cells by protecting complement component C3b against inactivation. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9144–9148. doi: 10.1073/pnas.83.23.9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam M., Welt S., Old L. J., Vogel C. W. A molecular mechanism of complement resistance of human melanoma cells. J Immunol. 1986 Apr 1;136(7):2534–2541. [PubMed] [Google Scholar]
- Ramanathan V. D., Curtis J., Turk J. L. Activation of the alternative pathway of complement by mycobacteria and cord factor. Infect Immun. 1980 Jul;29(1):30–35. doi: 10.1128/iai.29.1.30-35.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieger M., Trnka L., Skvor J., Mison P. Immunoprofile studies in patients with pulmonary tuberculosis. III. Study of haemolytic complement in serum and phagocytic activity of blood neutrophils. Scand J Respir Dis. 1979 Aug;60(4):172–175. [PubMed] [Google Scholar]
- Rook G. A. Progress in the immunology of the mycobacterioses. Clin Exp Immunol. 1987 Jul;69(1):1–9. [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Gordon S. Expression of a mannosyl-fucosyl receptor for endocytosis on cultured primary macrophages and their hybrids. J Cell Biol. 1982 Apr;93(1):49–56. doi: 10.1083/jcb.93.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel C. W., Müller-Eberhard H. J. Cobra venom factor: improved method for purification and biochemical characterization. J Immunol Methods. 1984 Oct 12;73(1):203–220. doi: 10.1016/0022-1759(84)90045-0. [DOI] [PubMed] [Google Scholar]
- Young L. S., Inderlied C. B., Berlin O. G., Gottlieb M. S. Mycobacterial infections in AIDS patients, with an emphasis on the Mycobacterium avium complex. Rev Infect Dis. 1986 Nov-Dec;8(6):1024–1033. doi: 10.1093/clinids/8.6.1024. [DOI] [PubMed] [Google Scholar]
- Zakowski P., Fligiel S., Berlin G. W., Johnson L., Jr Disseminated Mycobacterium avium-intracellulare infection in homosexual men dying of acquired immunodeficiency. JAMA. 1982 Dec 10;248(22):2980–2982. doi: 10.1001/jama.1982.03330220024029. [DOI] [PubMed] [Google Scholar]


