Abstract
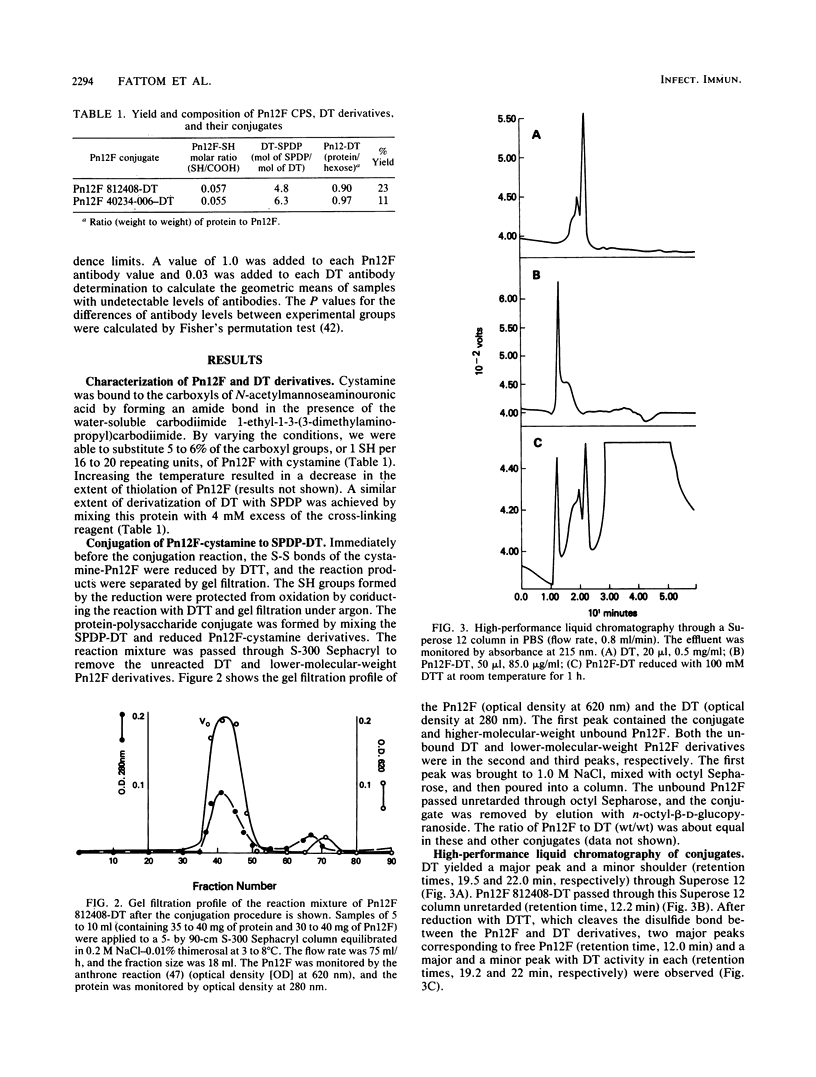
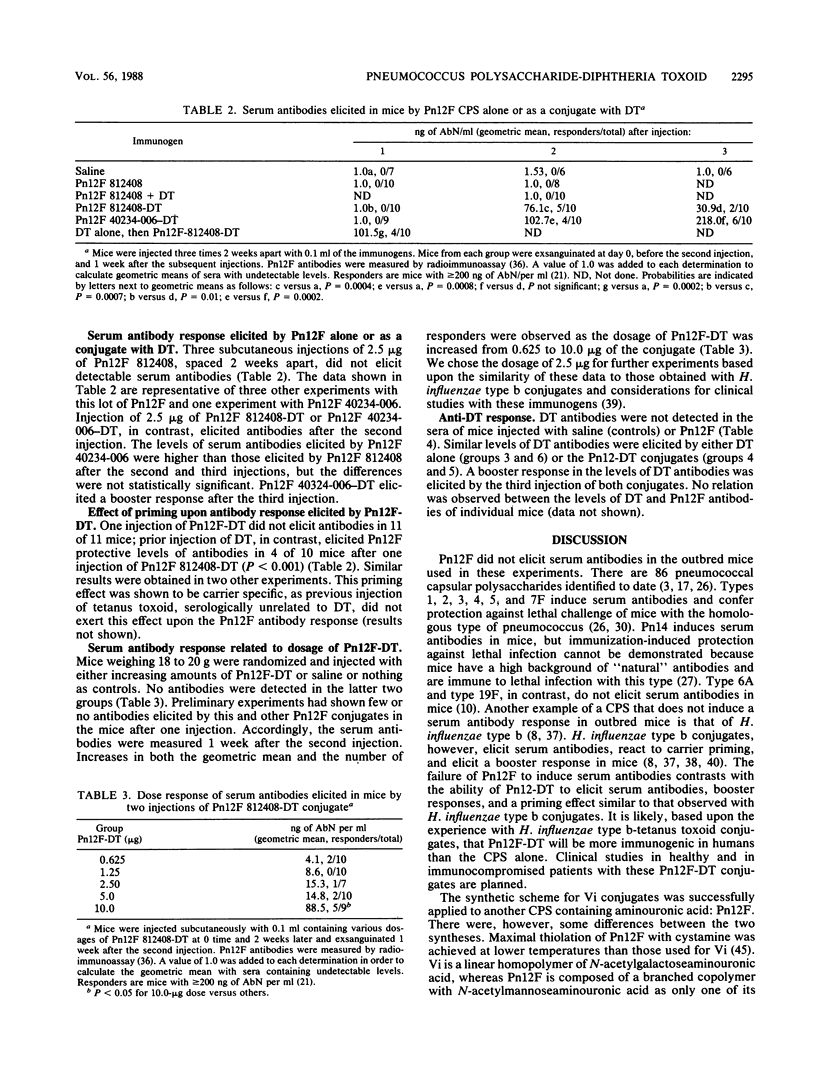
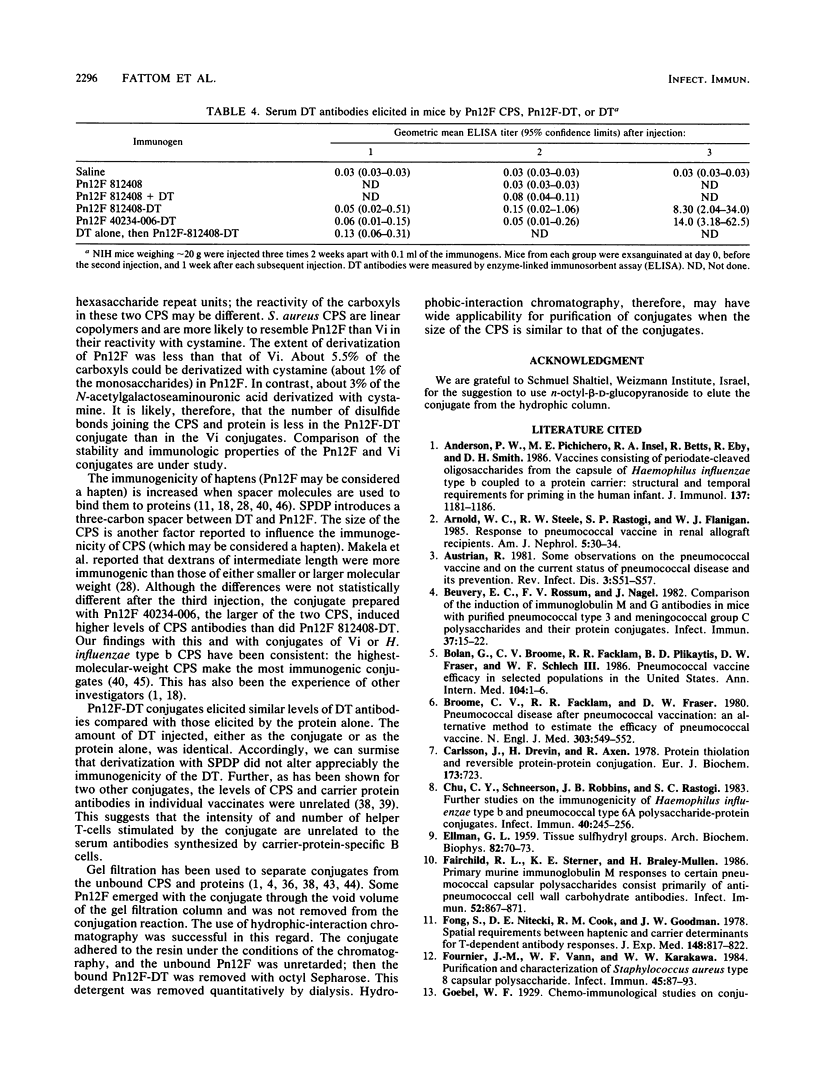
A scheme for the synthesis and purification of conjugates, composed of the type 12F capsular polysaccharide of Streptococcus pneumoniae (Pn12F) and diphtheria toxoid, is described. The scheme is a modification of that described previously for the Vi capsular polysaccharide of Salmonella typhi, a linear homopolymer of N-acetylgalactoseaminouronic acid (S. C. Szu, A. L. Stone, J. D. Robbins, R. Schneerson, and J. B. Robbins, J. Exp. Med. 166:1510-1524, 1986). Pn12F is a branched-chain copolymer composed of a hexasaccharide repeating unit containing an aminouronic acid, N-acetylmannoseaminouronic acid (K. Leontein, B. Lindberg, and J. Lonngren, Can. J. Chem. 59:2081-2085, 1981). Sulfhydryl groups were introduced into Pn12F by forming an amide bond between cystamine and carboxyl groups of N-acetylmannoseaminouronic acid in the presence of a carbodiimide. The disulfide moiety of cystamine was reduced to form the cysteamine derivative of Pn12F which was, in turn, covalently bound to diphtheria toxoid by using the heterobifunctional linker N-succinimidyl-3-(2-pyridylthio)propionate. Unbound, high-molecular-weight Pn12F was removed from the conjugate by hydrophobic interaction chromatography through octyl Sepharose by using n-octyl-beta-D-glucopyranoside as the eluent. In young outbred mice, Pn12F did not elicit detectable serum antibodies. Pn12F-diphtheria toxoid, in contrast, elicited antibodies after two injections and had T-cell-dependent properties as evidenced by a response to priming and by its ability to elicit booster responses. This scheme seems applicable to the synthesis of conjugates with other capsular polysaccharides containing aminouronic acids. Clinical evaluation of Pn12F-diphtheria toxoid conjugates in healthy and in immunocompromised hosts is planned.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. W., Pichichero M. E., Insel R. A., Betts R., Eby R., Smith D. H. Vaccines consisting of periodate-cleaved oligosaccharides from the capsule of Haemophilus influenzae type b coupled to a protein carrier: structural and temporal requirements for priming in the human infant. J Immunol. 1986 Aug 15;137(4):1181–1186. [PubMed] [Google Scholar]
- Arnold W. C., Steele R. W., Rastogi S. P., Flanigan W. J. Response to pneumococcal vaccine in renal allograft recipients. Am J Nephrol. 1985;5(1):30–34. doi: 10.1159/000166899. [DOI] [PubMed] [Google Scholar]
- Beuvery E. C., van Rossum F., Nagel J. Comparison of the induction of immunoglobulin M and G antibodies in mice with purified pneumococcal type 3 and meningococcal group C polysaccharides and their protein conjugates. Infect Immun. 1982 Jul;37(1):15–22. doi: 10.1128/iai.37.1.15-22.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolan G., Broome C. V., Facklam R. R., Plikaytis B. D., Fraser D. W., Schlech W. F., 3rd Pneumococcal vaccine efficacy in selected populations in the United States. Ann Intern Med. 1986 Jan;104(1):1–6. doi: 10.7326/0003-4819-104-1-1. [DOI] [PubMed] [Google Scholar]
- Broome C. V., Facklam R. R., Fraser D. W. Pneumococcal disease after pneumococcal vaccination: an alternative method to estimate the efficacy of pneumococcal vaccine. N Engl J Med. 1980 Sep 4;303(10):549–552. doi: 10.1056/NEJM198009043031003. [DOI] [PubMed] [Google Scholar]
- Carlsson J., Drevin H., Axén R. Protein thiolation and reversible protein-protein conjugation. N-Succinimidyl 3-(2-pyridyldithio)propionate, a new heterobifunctional reagent. Biochem J. 1978 Sep 1;173(3):723–737. doi: 10.1042/bj1730723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Schneerson R., Robbins J. B., Rastogi S. C. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun. 1983 Apr;40(1):245–256. doi: 10.1128/iai.40.1.245-256.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Fairchild R. L., Sterner K. E., Braley-Mullen H. Primary murine immunoglobulin M responses to certain pneumococcal capsular polysaccharides consist primarily of anti-pneumococcal cell wall carbohydrate antibodies. Infect Immun. 1986 Jun;52(3):867–871. doi: 10.1128/iai.52.3.867-871.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong S., Nitecki D. E., Cook R. M., Goodman J. W. Spatial requirements between haptenic and carrier determinants for T-dependent antibody responses. J Exp Med. 1978 Sep 1;148(3):817–822. doi: 10.1084/jem.148.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier J. M., Vann W. F., Karakawa W. W. Purification and characterization of Staphylococcus aureus type 8 capsular polysaccharide. Infect Immun. 1984 Jul;45(1):87–93. doi: 10.1128/iai.45.1.87-93.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilleman M. R., Carlson A. J., Jr, McLean A. A., Vella P. P., Weibel R. E., Woodhour A. F. Streptococcus pneumoniae polysaccharide vaccine: age and dose responses, safety, persistence of antibody, revaccination, and simultaneous administration of pneumococcal and influenza vaccines. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S31–S42. doi: 10.1093/clinids/3.supplement_1.s31. [DOI] [PubMed] [Google Scholar]
- Jörbeck H. J., Svenson S. B., Lindberg A. A. Artificial Salmonella vaccines: Salmonella typhimurium O-antigen-specific oligosaccharide-protein conjugates elicit opsonizing antibodies that enhance phagocytosis. Infect Immun. 1981 May;32(2):497–502. doi: 10.1128/iai.32.2.497-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu T., Lawn C. Y., Amsden A., Leskowitz S. Hapten-specific T cell response to azobenzenearsonate-N-acetyl-L-tyrosine in the Lewis rat. III. Effects of peptide-spacer structure on eliciting ABA-specific helper activity with TNP-haptened ABA-peptide-Ficoll. J Immunol. 1983 Feb;130(2):586–589. [PubMed] [Google Scholar]
- LANDY M. Studies on Vi antigen. VII. Characteristics of the immune response in the mouse. Am J Hyg. 1957 Jan;65(1):81–93. doi: 10.1093/oxfordjournals.aje.a119858. [DOI] [PubMed] [Google Scholar]
- Landesman S. H., Schiffman G. Assessment of the antibody response to pneumococcal vaccine in high-risk populations. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S184–S197. doi: 10.1093/clinids/3.supplement_1.s184. [DOI] [PubMed] [Google Scholar]
- Lee C. J., Lin K. T. Studies on vaccine control and immunogenicity of polysaccharides of Streptococcus pneumoniae. Rev Infect Dis. 1981 Mar-Apr;3 (Suppl):S51–S60. doi: 10.1093/clinids/3.supplement_1.s51. [DOI] [PubMed] [Google Scholar]
- Leontein K., Lindberg B., Lönngren J., Carlo D. J. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 12A. Carbohydr Res. 1983 Apr 1;114(2):257–266. doi: 10.1016/0008-6215(83)88192-0. [DOI] [PubMed] [Google Scholar]
- Liau D. F., Melly M. A., Hash J. H. Surface polysaccharide from Staphylococcus aureus M that contains taurine, D-aminogalacturonic acid, and D-fucosamine. J Bacteriol. 1974 Sep;119(3):913–922. doi: 10.1128/jb.119.3.913-922.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkelä O., Pasanen V. J., Sarvas H., Lehtonen M. A gene of the immunoglobulin H-chain cluster controls the murine antibody response to pneumococcal polysaccharide type 14. Scand J Immunol. 1980;12(2):155–158. doi: 10.1111/j.1365-3083.1980.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Mäkelä O., Péterfy F., Outschoorn I. G., Richter A. W., Seppälä I. Immunogenic properties of alpha (1----6) dextran, its protein conjugates, and conjugates of its breakdown products in mice. Scand J Immunol. 1984 Jun;19(6):541–550. doi: 10.1111/j.1365-3083.1984.tb00965.x. [DOI] [PubMed] [Google Scholar]
- Oravec L. S., Lee C. J., Hoppe P. A., Santos C. V. Detection of blood group A-like substance in bacterial and viral vaccines by countercurrent immunoelectrophoresis using Helix pomatia lectin. J Biol Stand. 1984;12(2):159–166. doi: 10.1016/s0092-1157(84)80049-9. [DOI] [PubMed] [Google Scholar]
- Reingold A. L., Broome C. V., Hightower A. W., Ajello G. W., Bolan G. A., Adamsbaum C., Jones E. E., Phillips C., Tiendrebeogo H., Yada A. Age-specific differences in duration of clinical protection after vaccination with meningococcal polysaccharide A vaccine. Lancet. 1985 Jul 20;2(8447):114–118. doi: 10.1016/s0140-6736(85)90224-7. [DOI] [PubMed] [Google Scholar]
- Robbins J. B., Austrian R., Lee C. J., Rastogi S. C., Schiffman G., Henrichsen J., Mäkelä P. H., Broome C. V., Facklam R. R., Tiesjema R. H. Considerations for formulating the second-generation pneumococcal capsular polysaccharide vaccine with emphasis on the cross-reactive types within groups. J Infect Dis. 1983 Dec;148(6):1136–1159. doi: 10.1093/infdis/148.6.1136. [DOI] [PubMed] [Google Scholar]
- Robbins J. B. Vaccines for the prevention of encapsulated bacterial diseases: current status, problems and prospects for the future. Immunochemistry. 1978 Nov;15(10-11):839–854. doi: 10.1016/0161-5890(78)90117-7. [DOI] [PubMed] [Google Scholar]
- Schiffman G., Douglas R. M., Bonner M. J., Robbins M., Austrian R. A radioimmunoassay for immunologic phenomena in pneumococcal disease and for the antibody response to pneumococcal vaccines. I. Method for the radioimmunoassay of anticapsular antibodies and comparison with other techniques. J Immunol Methods. 1980;33(2):133–144. doi: 10.1016/s0022-1759(80)80004-4. [DOI] [PubMed] [Google Scholar]
- Schneerson R., Barrera O., Sutton A., Robbins J. B. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J Exp Med. 1980 Aug 1;152(2):361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Chu C., Sutton A., Vann W., Vickers J. C., London W. T., Curfman B., Hardegree M. C., Shiloach J. Serum antibody responses of juvenile and infant rhesus monkeys injected with Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-protein conjugates. Infect Immun. 1984 Sep;45(3):582–591. doi: 10.1128/iai.45.3.582-591.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneerson R., Robbins J. B., Parke J. C., Jr, Bell C., Schlesselman J. J., Sutton A., Wang Z., Schiffman G., Karpas A., Shiloach J. Quantitative and qualitative analyses of serum antibodies elicited in adults by Haemophilus influenzae type b and pneumococcus type 6A capsular polysaccharide-tetanus toxoid conjugates. Infect Immun. 1986 May;52(2):519–528. doi: 10.1128/iai.52.2.519-528.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Svenson S. B., Nurminen M., Lindberg A. A. Artificial Salmonella vaccines: O-antigenic oligosaccharide-protein conjugates induce protection against infection with Salmonella typhimurium. Infect Immun. 1979 Sep;25(3):863–872. doi: 10.1128/iai.25.3.863-872.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Schneerson R., Robbins J. B. Rabbit antibodies to the cell wall polysaccharide of Streptococcus pneumoniae fail to protect mice from lethal challenge with encapsulated pneumococci. Infect Immun. 1986 Nov;54(2):448–455. doi: 10.1128/iai.54.2.448-455.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szu S. C., Stone A. L., Robbins J. D., Schneerson R., Robbins J. B. Vi capsular polysaccharide-protein conjugates for prevention of typhoid fever. Preparation, characterization, and immunogenicity in laboratory animals. J Exp Med. 1987 Nov 1;166(5):1510–1524. doi: 10.1084/jem.166.5.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson W. A., Greenwood B. M. Impairment of the immune response to vaccination after acute malaria. Lancet. 1978 Jun 24;1(8078):1328–1329. doi: 10.1016/s0140-6736(78)92403-0. [DOI] [PubMed] [Google Scholar]
- Winston D. J., Ho W. G., Schiffman G., Champlin R. E., Feig S. A., Gale R. P. Pneumococcal vaccination of recipients of bone marrow transplants. Arch Intern Med. 1983 Sep;143(9):1735–1737. [PubMed] [Google Scholar]
- Winston D. J., Schiffman G., Wang D. C., Feig S. A., Lin C. H., Marso E. L., Ho W. G., Young L. S., Gale R. P. Pneumococcal infections after human bone-marrow transplantation. Ann Intern Med. 1979 Dec;91(6):835–841. doi: 10.7326/0003-4819-91-6-835. [DOI] [PubMed] [Google Scholar]



