Abstract
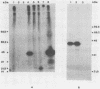
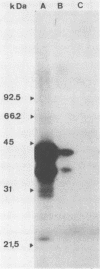
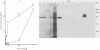
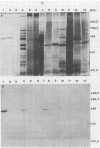
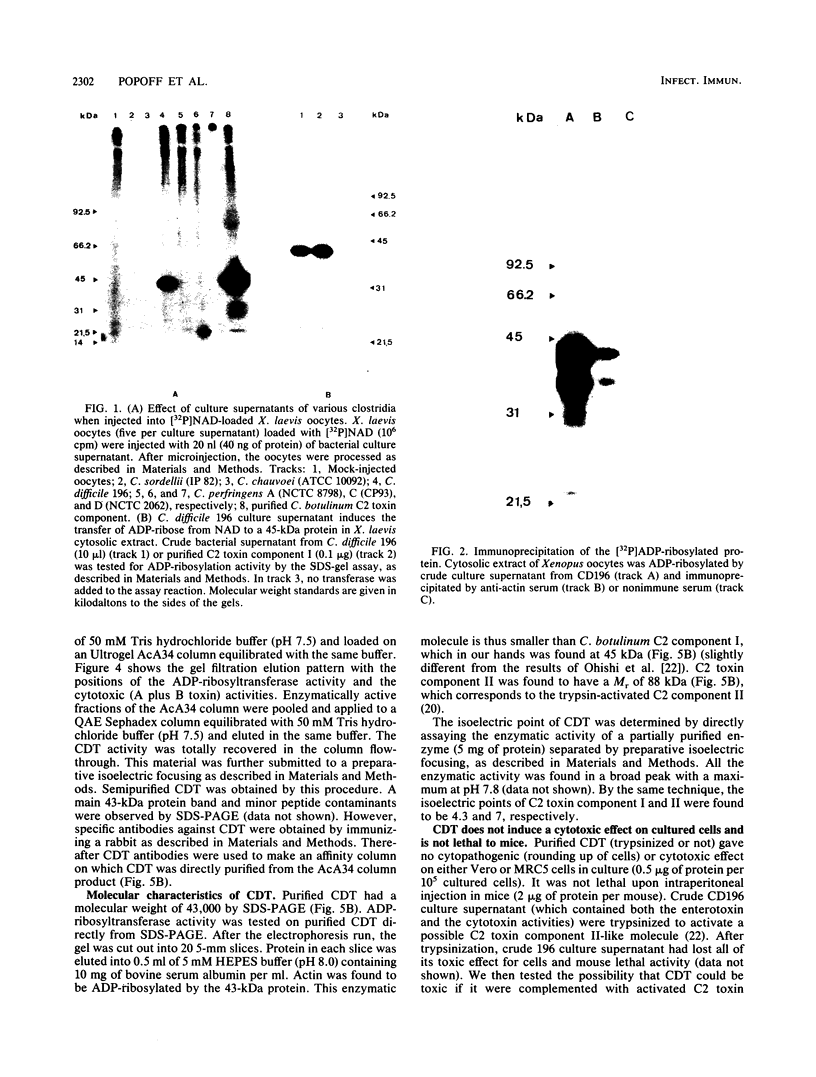
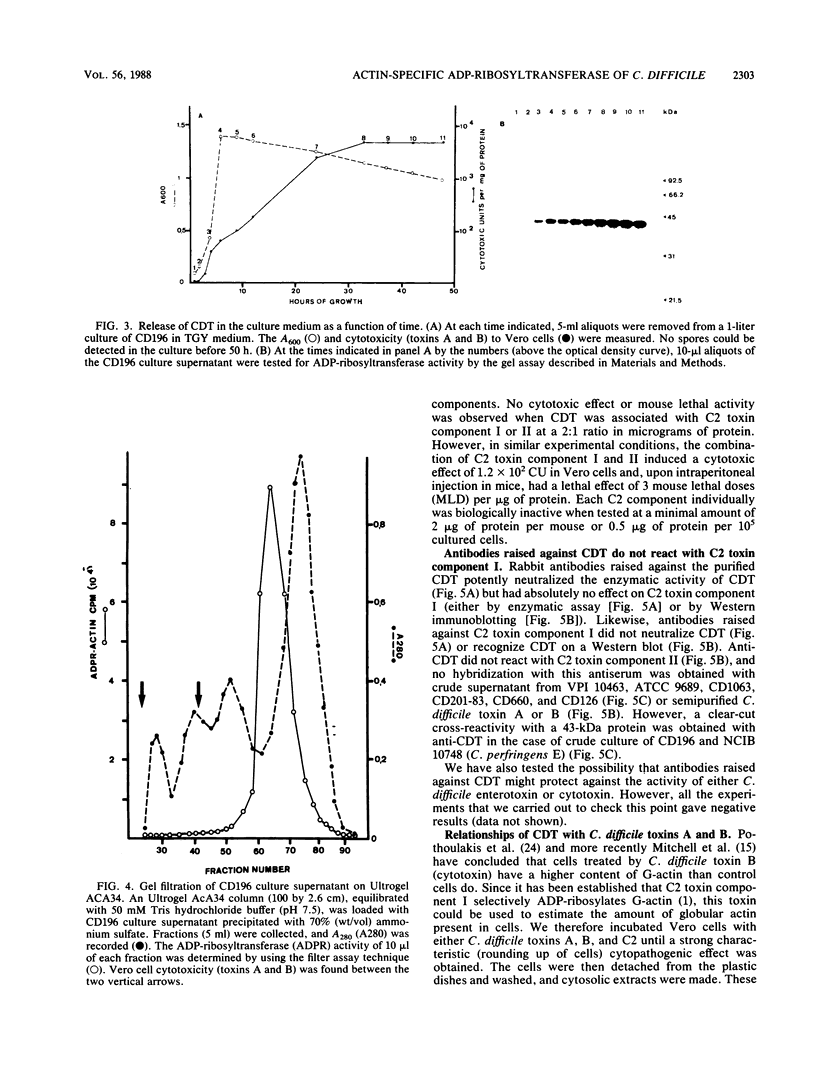
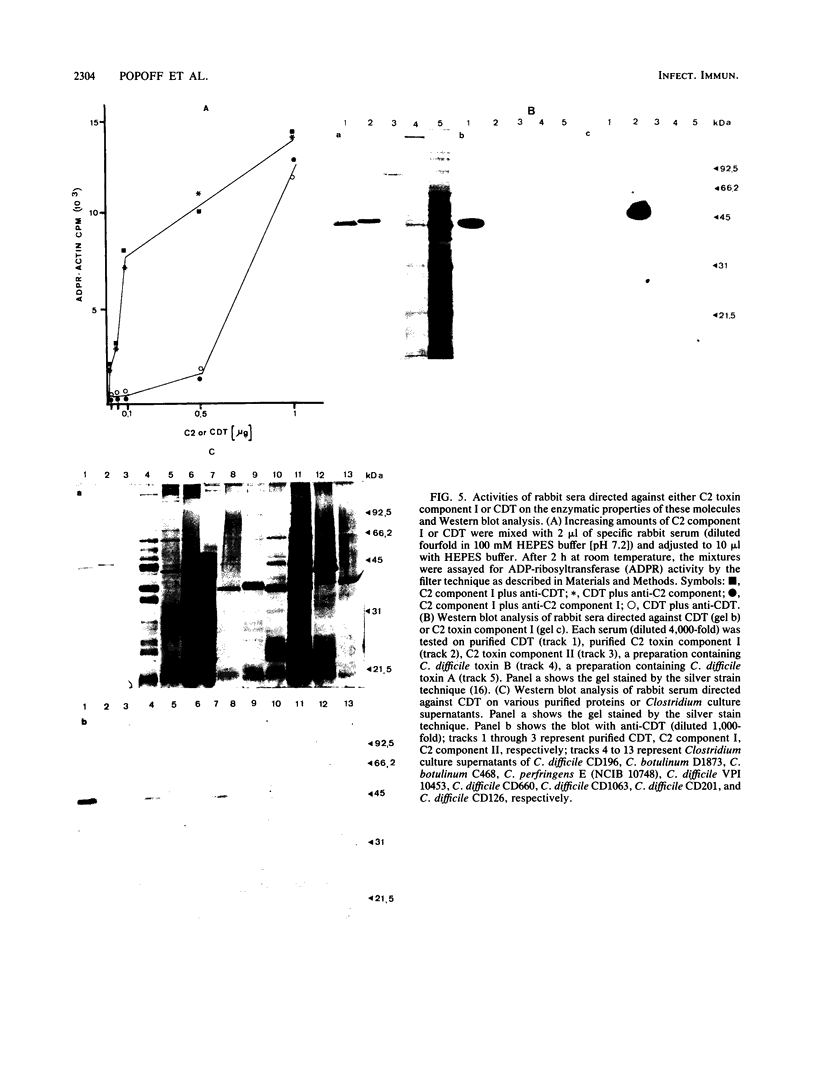
By screening possible ADP-ribosyltransferase activities in culture supernatants from various Clostridium species, we have found one Clostridium difficile strain (CD196) (isolated in our laboratory) that is able to produce, in addition to toxins A and B, a new ADP-ribosyltransferase that was shown to covalently modify cell actin as Clostridium botulinum C2 or Clostridium perfringens E iota toxins do. The molecular weight of the CD196 ADP-ribosyltransferase (CDT) was determined to be 43 kilodaltons, and its isoelectric point was 7.8. No cytotoxic activity on Vero cells or lethal activity upon injection in mice was associated with this enzyme. CDT was neither related to C. difficile A or B toxins nor to C. botulinum C2 toxin component I. However, Vero cells cultivated in the presence of C. difficile B toxin had a lower amount of actin able to be ADP-ribosylated by CDT or C2 toxin in vitro. Antibodies raised against CDT reacted by immunoblot analysis with a 43-kilodalton protein of C. perfringens type E culture supernatant producing the iota toxin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aktories K., Bärmann M., Ohishi I., Tsuyama S., Jakobs K. H., Habermann E. Botulinum C2 toxin ADP-ribosylates actin. Nature. 1986 Jul 24;322(6077):390–392. doi: 10.1038/322390a0. [DOI] [PubMed] [Google Scholar]
- Borriello S. P., Carman R. J. Association of iota-like toxin and Clostridium spiroforme with both spontaneous and antibiotic-associated diarrhea and colitis in rabbits. J Clin Microbiol. 1983 Mar;17(3):414–418. doi: 10.1128/jcm.17.3.414-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Lazarides E. ADP-ribosylation of the Mr 83,000 stress-inducible and glucose-regulated protein in avian and mammalian cells: modulation by heat shock and glucose starvation. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4664–4668. doi: 10.1073/pnas.80.15.4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donta S. T., Sullivan N., Wilkins T. D. Differential effects of Clostridium difficile toxins on tissue-cultured cells. J Clin Microbiol. 1982 Jun;15(6):1157–1158. doi: 10.1128/jcm.15.6.1157-1158.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin I., Thelestam M. ADP-ribosylation in cultured cells treated with Clostridium difficile toxin B. Biochem Biophys Res Commun. 1986 Aug 29;139(1):64–70. doi: 10.1016/s0006-291x(86)80080-8. [DOI] [PubMed] [Google Scholar]
- Godeau F., Boquet P., Schorderet-Slatkine S., Schorderet M., Baulieu E. E. Studies of microbial toxins in Xenopus laevis oocytes. Exp Cell Res. 1980 Sep;129(1):133–137. doi: 10.1016/0014-4827(80)90338-9. [DOI] [PubMed] [Google Scholar]
- Guyonnet F., Plommet M. Hémolysine gamma de staphylococcus aureus: purification et propriétés. Ann Inst Pasteur (Paris) 1970 Jan;118(1):19–33. [PubMed] [Google Scholar]
- Iwasaki M., Ohishi I., Sakaguchi G. Evidence that botulinum C2 toxin has two dissimilar components. Infect Immun. 1980 Aug;29(2):390–394. doi: 10.1128/iai.29.2.390-394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan H. C., Wilkins T. D. Purification of Clostridium difficile toxin A by affinity chromatography on immobilized thyroglobulin. Infect Immun. 1987 Aug;55(8):1873–1877. doi: 10.1128/iai.55.8.1873-1877.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. J., Laughon B. E., Lin S. Biochemical studies on the effect of Clostridium difficile toxin B on actin in vivo and in vitro. Infect Immun. 1987 Jul;55(7):1610–1615. doi: 10.1128/iai.55.7.1610-1615.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Serikawa T., Yamakawa K., Nishida S., Kozaki S., Sakaguchi G. Sporulation and C2 toxin production by Clostridium botulinum type C strains producing no C1 toxin. Microbiol Immunol. 1978;22(10):591–596. doi: 10.1111/j.1348-0421.1978.tb00409.x. [DOI] [PubMed] [Google Scholar]
- Noda M., Hirayama T., Kato I., Matsuda F. Crystallization and properties of staphylococcal leukocidin. Biochim Biophys Acta. 1980 Nov 17;633(1):33–44. doi: 10.1016/0304-4165(80)90035-5. [DOI] [PubMed] [Google Scholar]
- Ohishi I. Activation of botulinum C2 toxin by trypsin. Infect Immun. 1987 Jun;55(6):1461–1465. doi: 10.1128/iai.55.6.1461-1465.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Iwasaki M., Sakaguchi G. Purification and characterization of two components of botulinum C2 toxin. Infect Immun. 1980 Dec;30(3):668–673. doi: 10.1128/iai.30.3.668-673.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I. Response of mouse intestinal loop to botulinum C2 toxin: enterotoxic activity induced by cooperation of nonlinked protein components. Infect Immun. 1983 May;40(2):691–695. doi: 10.1128/iai.40.2.691-695.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi I., Tsuyama S. ADP-ribosylation of nonmuscle actin with component I of C2 toxin. Biochem Biophys Res Commun. 1986 Apr 29;136(2):802–806. doi: 10.1016/0006-291x(86)90511-5. [DOI] [PubMed] [Google Scholar]
- Popoff M. R. Purification and characterization of Clostridium sordellii lethal toxin and cross-reactivity with Clostridium difficile cytotoxin. Infect Immun. 1987 Jan;55(1):35–43. doi: 10.1128/iai.55.1.35-43.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pothoulakis C., Barone L. M., Ely R., Faris B., Clark M. E., Franzblau C., LaMont J. T. Purification and properties of Clostridium difficile cytotoxin B. J Biol Chem. 1986 Jan 25;261(3):1316–1321. [PubMed] [Google Scholar]
- Reuner K. H., Presek P., Boschek C. B., Aktories K. Botulinum C2 toxin ADP-ribosylates actin and disorganizes the microfilament network in intact cells. Eur J Cell Biol. 1987 Feb;43(1):134–140. [PubMed] [Google Scholar]
- Roa M., Boquet P. Interaction of tetanus toxin with lipid vesicles at low pH. Protection of specific polypeptides against proteolysis. J Biol Chem. 1985 Jun 10;260(11):6827–6835. [PubMed] [Google Scholar]
- Robertson D. L., Leppla S. H. Molecular cloning and expression in Escherichia coli of the lethal factor gene of Bacillus anthracis. Gene. 1986;44(1):71–78. doi: 10.1016/0378-1119(86)90044-2. [DOI] [PubMed] [Google Scholar]
- Rubin E. J., Gill D. M., Boquet P., Popoff M. R. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988 Jan;8(1):418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L. L. Molecular basis for the pharmacological actions of Clostridium botulinum type C2 toxin. J Pharmacol Exp Ther. 1984 Sep;230(3):665–669. [PubMed] [Google Scholar]
- Simpson L. L. Molecular pharmacology of botulinum toxin and tetanus toxin. Annu Rev Pharmacol Toxicol. 1986;26:427–453. doi: 10.1146/annurev.pa.26.040186.002235. [DOI] [PubMed] [Google Scholar]
- Simpson L. L., Stiles B. G., Zepeda H. H., Wilkins T. D. Molecular basis for the pathological actions of Clostridium perfringens iota toxin. Infect Immun. 1987 Jan;55(1):118–122. doi: 10.1128/iai.55.1.118-122.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiles B. G., Wilkins T. D. Clostridium perfringens iota toxin: synergism between two proteins. Toxicon. 1986;24(8):767–773. doi: 10.1016/0041-0101(86)90101-7. [DOI] [PubMed] [Google Scholar]
- Stiles B. G., Wilkins T. D. Purification and characterization of Clostridium perfringens iota toxin: dependence on two nonlinked proteins for biological activity. Infect Immun. 1986 Dec;54(3):683–688. doi: 10.1128/iai.54.3.683-688.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan N. M., Pellett S., Wilkins T. D. Purification and characterization of toxins A and B of Clostridium difficile. Infect Immun. 1982 Mar;35(3):1032–1040. doi: 10.1128/iai.35.3.1032-1040.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor N. S., Thorne G. M., Bartlett J. G. Comparison of two toxins produced by Clostridium difficile. Infect Immun. 1981 Dec;34(3):1036–1043. doi: 10.1128/iai.34.3.1036-1043.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelestam M., Brönnegård M. Interaction of cytopathogenic toxin from Clostridium difficile with cells in tissue culture. Scand J Infect Dis Suppl. 1980;(Suppl 22):16–29. [PubMed] [Google Scholar]
- Vandekerckhove J., Schering B., Bärmann M., Aktories K. Clostridium perfringens iota toxin ADP-ribosylates skeletal muscle actin in Arg-177. FEBS Lett. 1987 Dec 10;225(1-2):48–52. doi: 10.1016/0014-5793(87)81129-8. [DOI] [PubMed] [Google Scholar]
- Wilkins T. D. Role of Clostridium difficile toxins in disease. Gastroenterology. 1987 Aug;93(2):389–391. doi: 10.1016/0016-5085(87)91031-6. [DOI] [PubMed] [Google Scholar]