Abstract
Although antigen-reactive T lymphocytes play a central role in the host response to Histoplasma capsulatum, little is known of the nature of Histoplasma antigens recognized by these cells in vitro. Employing a murine T-cell line and two clones that are reactive with histoplasmin, we examined whether activation of T cells by histoplasmin required the presence of carbohydrate or protein moieties. The approach taken was to modify carbohydrate or protein molecules in histoplasmin by chemical or enzymatic digestion or by lectin adsorption. In parallel, antigen was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis to correlate alterations in functional activity with changes in the electrophoretic appearance of histoplasmin. Treatment of histoplasmin with periodate (0.1 M, 0.05 M, and 0.01 M) or with the endoglycosidases N-glycanase and endoglycosidase H sharply diminished the capacity of histoplasmin to trigger responses by T cells. Reactivity of T cells to histoplasmin that had been adsorbed with lectins binding mannose, glucose, or galactose was reduced by greater than 70%; conversely, the responses by T cells to antigen that had been adsorbed with lectins specific for fucose, N-acetylgalactosamine, or N-acetylglucosamine ranged from 82 to 91% of that to control antigen. Proliferative responses by T cells to histoplasmin that had been digested with chymotrypsin, protease, or trypsin were 2 to 43% of control values. The electrophoretic appearance of histoplasmin was modified by some but not all of the treatments. Partially purified H and M antigens triggered proliferation of T cells. Thus, both carbohydrates and proteins must be present to induce optimal responses by T cells. A portion of the carbohydrates is N linked to proteins, and alpha-D-mannose (or alpha-D-glucose) and beta-D-galactose are the sugar ligands of carbohydrate-containing antigens.
Full text
PDF

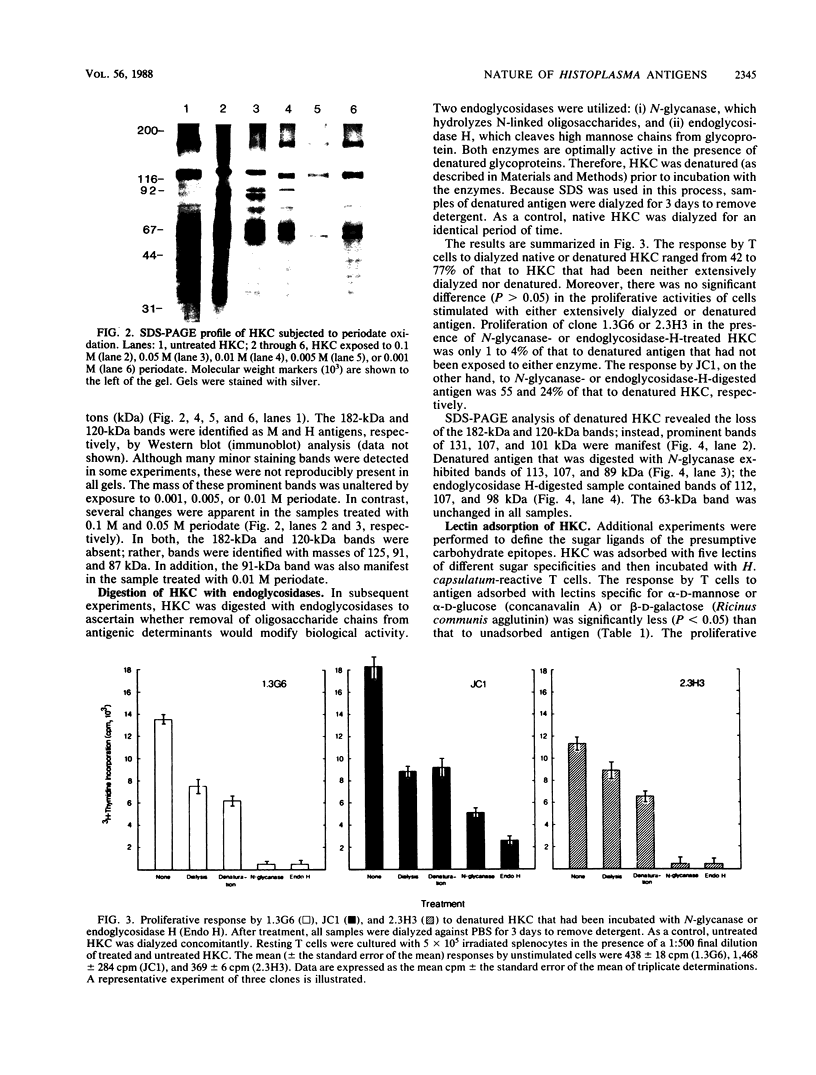




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azuma I., Kanetsuna F., Tanaka Y., Yamamura Y., Carbonell L. M. Chemical and immunological properties of galactomannans obtained from Histoplasma duboisii, Histoplasma capsulatum, Paracoccidioides brasiliensis and Blasomyces dermatitidis. Mycopathol Mycol Appl. 1974 Oct 15;54(1):111–125. doi: 10.1007/BF02055979. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bradley G., Pine L., Reeves M. W., Moss C. W. Purification, composition, and serological characterization of histoplasmin-H and M antigens. Infect Immun. 1974 May;9(5):870–880. doi: 10.1128/iai.9.5.870-880.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R. A. Immunologic studies of patients with histoplasmosis. Am Rev Respir Dis. 1979 Jul;120(1):143–149. doi: 10.1164/arrd.1979.120.1.143. [DOI] [PubMed] [Google Scholar]
- Deepe G. S., Jr, Smith J. G., Sonnenfeld G., Denman D., Bullock W. E. Development and characterization of Histoplasma capsulatum-reactive murine T-cell lines and clones. Infect Immun. 1986 Dec;54(3):714–722. doi: 10.1128/iai.54.3.714-722.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durandy A., Fischer A., Charron D., Griscelli C. Specific binding of antigen onto human T lymphocytes. J Clin Invest. 1986 May;77(5):1557–1564. doi: 10.1172/JCI112471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEINER D. C. Diagnosis of histoplasmosis using precipitin reactions in agargel. Pediatrics. 1958 Oct;22(4 Pt 1):616–627. [PubMed] [Google Scholar]
- Khardori N., Chaudhary S., McConnachie P., Tewari R. P. Characterization of lymphocytes responsible for protective immunity to histoplasmosis in mice. Mykosen. 1983 Oct;26(10):523–532. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markham R. B., Pier G. B., Goellner J. J., Mizel S. B. In vitro T cell-mediated killing of Pseudomonas aeruginosa. II. The role of macrophages and T cell subsets in T cell killing. J Immunol. 1985 Jun;134(6):4112–4117. [PubMed] [Google Scholar]
- Mustafa A. S., Gill H. K., Nerland A., Britton W. J., Mehra V., Bloom B. R., Young R. A., Godal T. Human T-cell clones recognize a major M. leprae protein antigen expressed in E. coli. Nature. 1986 Jan 2;319(6048):63–66. doi: 10.1038/319063a0. [DOI] [PubMed] [Google Scholar]
- Newberry W. M., Jr, Chandler J. W., Jr, Chin T. D., Kirkpatrick C. H. Immunology of the mycoses. I. Depressed lymphocyte transformation in chronic histoplasmosis. J Immunol. 1968 Feb;100(2):436–443. [PubMed] [Google Scholar]
- Reeves M. W., Pine L., Bradley G. Characterization and evaluation of a soluble antigen complex prepared from the yeast phase of Histoplasma capsulatum. Infect Immun. 1974 Jun;9(6):1033–1044. doi: 10.1128/iai.9.6.1033-1044.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHAW L. W., HOWELL A., Jr, WEISS E. S. Biological assay of lots of histoplasmin and the selection of a new working lot. Public Health Rep. 1950 May 5;65(18):583–609. [PMC free article] [PubMed] [Google Scholar]
- Sheppard H. W., Scott P. A., Dwyer D. M. Recognition of Leishmania donovani antigens by murine T lymphocyte lines and clones. Species cross-reactivity, functional correlates of cell-mediated immunity, and antigen characterization. J Immunol. 1983 Sep;131(3):1496–1503. [PubMed] [Google Scholar]
- Shevach E. M., Rosenthal A. S. Function of macrophages in antigen recognition by guinea pig T lymphocytes. II. Role of the macrophage in the regulation of genetic control of the immune response. J Exp Med. 1973 Nov 1;138(5):1213–1229. doi: 10.1084/jem.138.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprouse R. F. Determination of molecular weight, isoelectric point, and glycoprotein moiety for the principal skin test-reactive component of histoplasmin. Infect Immun. 1977 Jan;15(1):263–271. doi: 10.1128/iai.15.1.263-271.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang V. C., Hancock K., Maddison S. E., Beatty A. L., Moss D. M. Demonstration of species-specific and cross-reactive components of the adult microsomal antigens from Schistosoma mansoni and S. japonicum (MAMA and JAMA). J Immunol. 1984 May;132(5):2607–2613. [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Williams D. M., Graybill J. R., Drutz D. J. Histoplasma capsulatum infection in nude mice. Infect Immun. 1978 Sep;21(3):973–977. doi: 10.1128/iai.21.3.973-977.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu-Hsieh B., Howard D. H. Inhibition of growth of Histoplasma capsulatum by lymphokine-stimulated macrophages. J Immunol. 1984 May;132(5):2593–2597. [PubMed] [Google Scholar]
- Ziegler H. K., Unanue E. R. Decrease in macrophage antigen catabolism caused by ammonia and chloroquine is associated with inhibition of antigen presentation to T cells. Proc Natl Acad Sci U S A. 1982 Jan;79(1):175–178. doi: 10.1073/pnas.79.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler K., Unanue E. R. Identification of a macrophage antigen-processing event required for I-region-restricted antigen presentation to T lymphocytes. J Immunol. 1981 Nov;127(5):1869–1875. [PubMed] [Google Scholar]