Abstract
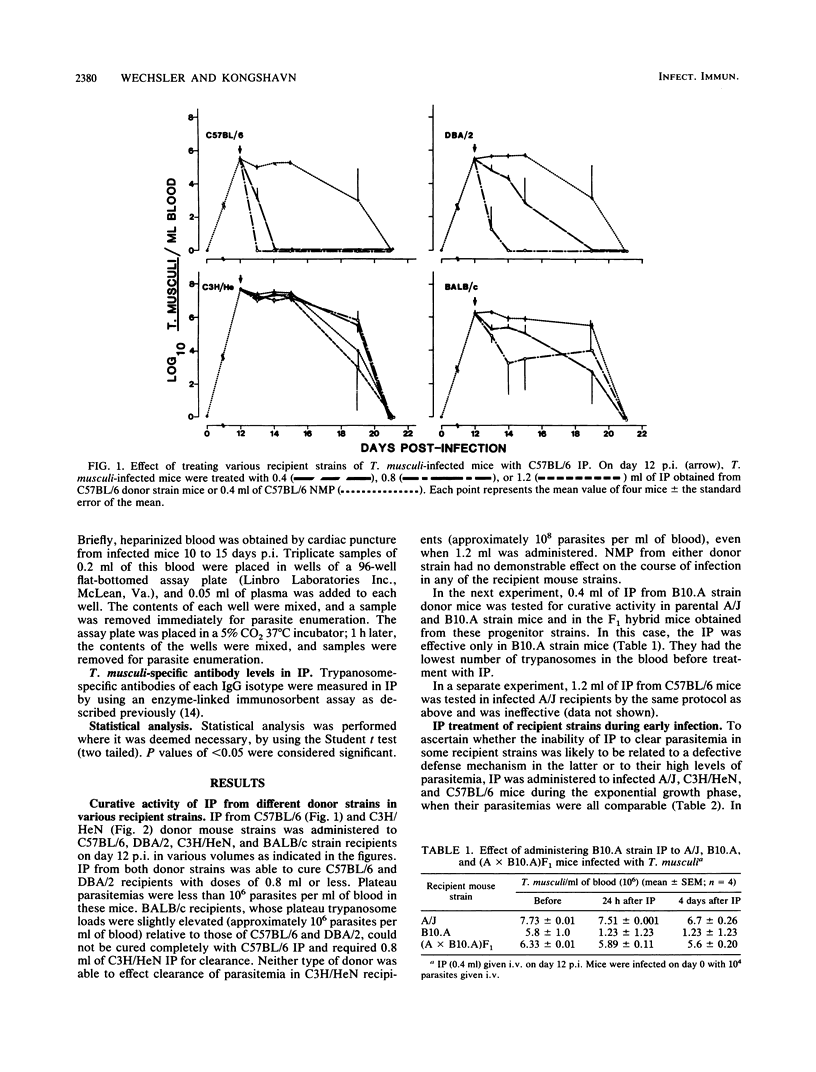
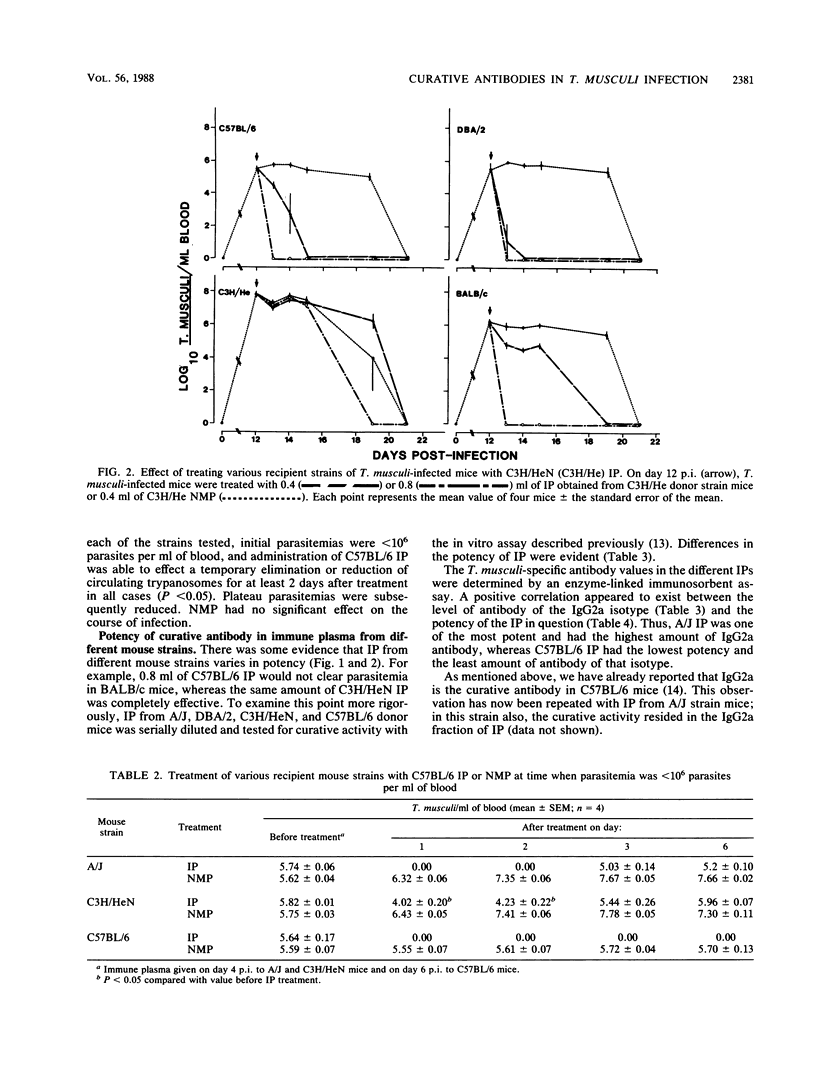
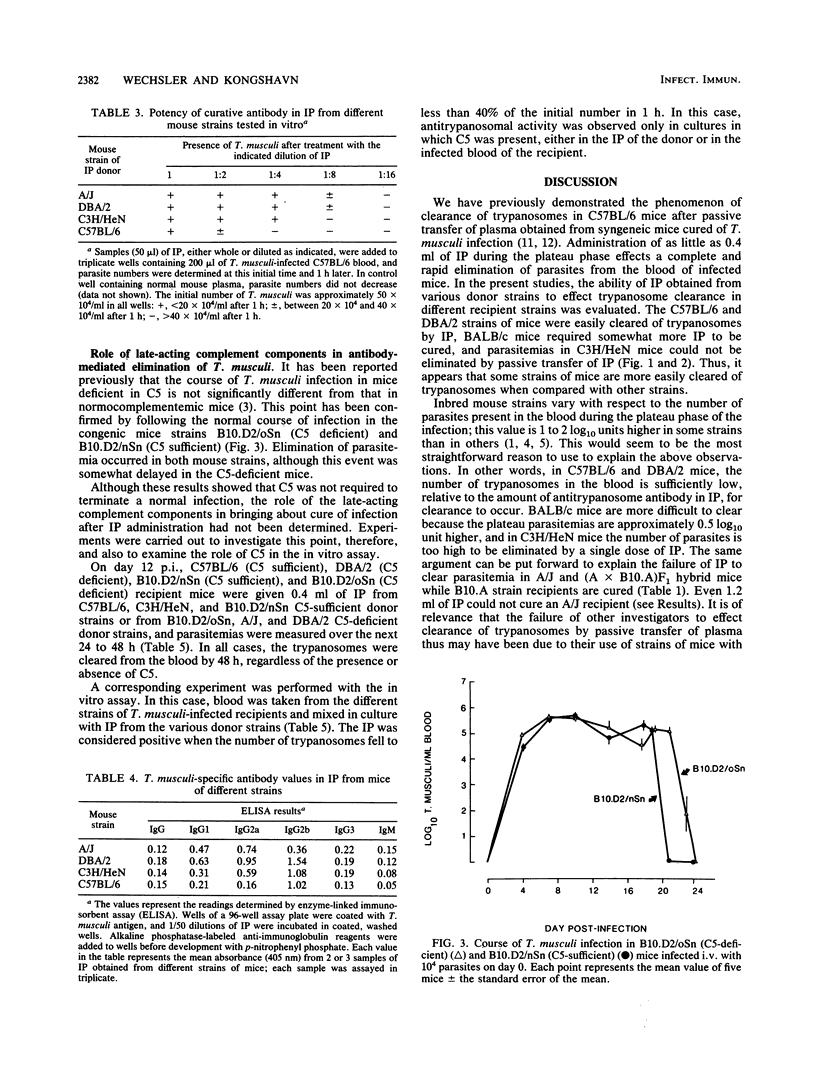
The ability of immune plasma (IP) taken from different donor strains of mice to cure Trypanosoma musculi infection in various recipient mouse strains, when given during the plateau phase of infection, was examined. C57BL/6, B10.A/SgSn, B10.D2/oSn, B10.D2/nSn, DBA/2, and BALB/c strains could be cured of parasitemia (giving 0.4 to 0.8 ml of IP per mouse), whereas A/J and C3H/HeN strains could not (giving up to 1.2 ml of IP per mouse). Noncure appeared to be associated with the high-plateau parasitemias (approximately 10(8] that developed in the latter strains since IP administered early in infection, when the parasite burden was similar to the plateau parasitemias (approximately 10(6] of strains that could be cured, was at least partially effective in A/J and C3H/HeN mice. The IP of any strain tested (C57BL/6, B10.D2/oSn, B10.D2/nSn, DBA/2, A/J, or C3H/HeN) could bring about elimination of trypanosomes in strains able to be cured. The potency of IP from different strains varied, being greater in the strains that developed higher-plateau parasitemias. Potency of IP appears to correlate positively with the titers of trypanosome-specific antibody of the immunoglobulin G2a isotype (the curative antibody). The role of the late-acting complement components was examined. In C5-deficient mice the course of infection was normal, although the elimination phase was delayed by a few days. Cure of parasitemia by IP administered during the plateau phase was equally effective in the presence or absence of C5 in either the donor or the recipient. When tested in vitro, however, IP only exhibited antitrypanosomal activity when added to infected blood taken from C5-sufficient strains of mice. We conclude that in vitro, under the conditions used in the assay, antibody-mediated destruction of the trypanosomes is brought about by complement-mediated lysis. This process, although it probably occurs to some extent, is unlikely to be the major mechanism of trypanosome elimination in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright J. W., Albright J. F. Differences in resistance to Trypanosoma musculi infection among strains of inbred mice. Infect Immun. 1981 Aug;33(2):364–371. doi: 10.1128/iai.33.2.364-371.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albright J. W., Albright J. F. The decline of immunological resistance of aging mice to Trypanosoma musculi. Mech Ageing Dev. 1982 Dec;20(4):315–330. doi: 10.1016/0047-6374(82)90099-9. [DOI] [PubMed] [Google Scholar]
- Jarvinen J. A., Dalmasso A. P. Trypanosoma musculi infections in normocomplementemic, C5-deficient, and C3-depleted mice. Infect Immun. 1977 May;16(2):557–563. doi: 10.1128/iai.16.2.557-563.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magluilo P., Viens P., Forget A. Immunosuppression during Trypanosoma musculi infection in inbred strains of mice. J Clin Lab Immunol. 1983 Mar;10(3):151–154. [PubMed] [Google Scholar]
- Olivier M., Tijssen P., Viens P. Participation of IgG2b antibodies in the initial control of Trypanosoma musculi infection. Parasite Immunol. 1986 Jan;8(1):27–29. doi: 10.1111/j.1365-3024.1986.tb00830.x. [DOI] [PubMed] [Google Scholar]
- Targett G. A., Viens P. The immunological response of CBA mice to Trypanosoma musculi: elimination of the parasite from the blood. Int J Parasitol. 1975 Apr;5(2):231–234. doi: 10.1016/0020-7519(75)90034-x. [DOI] [PubMed] [Google Scholar]
- Vargas F. D., Viens P., Kongshavn P. A. Trypanosoma musculi infection in B-cell-deficient mice. Infect Immun. 1984 Apr;44(1):162–167. doi: 10.1128/iai.44.1.162-167.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viens P., Targett G. A., Leuchars E., Davies A. J. The immunological response of CBA mice to Trypanosoma musculi. I. Initial control of the infection and the effect of T-cell deprivation. Clin Exp Immunol. 1974 Feb;16(2):279–294. [PMC free article] [PubMed] [Google Scholar]
- Vincendeau P., Daëron M., Daulouede S. Identification of antibody classes and Fc receptors responsible for phagocytosis of Trypanosoma musculi by mouse macrophages. Infect Immun. 1986 Sep;53(3):600–605. doi: 10.1128/iai.53.3.600-605.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Characterization of antibodies mediating protection and cure of Trypanosoma musculi infection in mice. Infect Immun. 1985 Jun;48(3):787–794. doi: 10.1128/iai.48.3.787-794.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Cure of Trypanosoma musculi infection by heat-labile activity in immune plasma. Infect Immun. 1984 Jun;44(3):756–759. doi: 10.1128/iai.44.3.756-759.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. Heat-labile IgG2a antibodies affect cure of Trypanosoma musculi infection in C57BL/6 mice. J Immunol. 1986 Nov 1;137(9):2968–2972. [PubMed] [Google Scholar]
- Wechsler D. S., Kongshavn P. A. In vitro assay for curative activity in blood of mice infected with Trypanosoma musculi. Infect Immun. 1986 Aug;53(2):240–244. doi: 10.1128/iai.53.2.240-244.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]



