Abstract
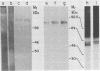
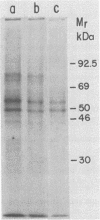
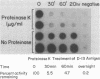
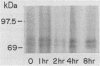
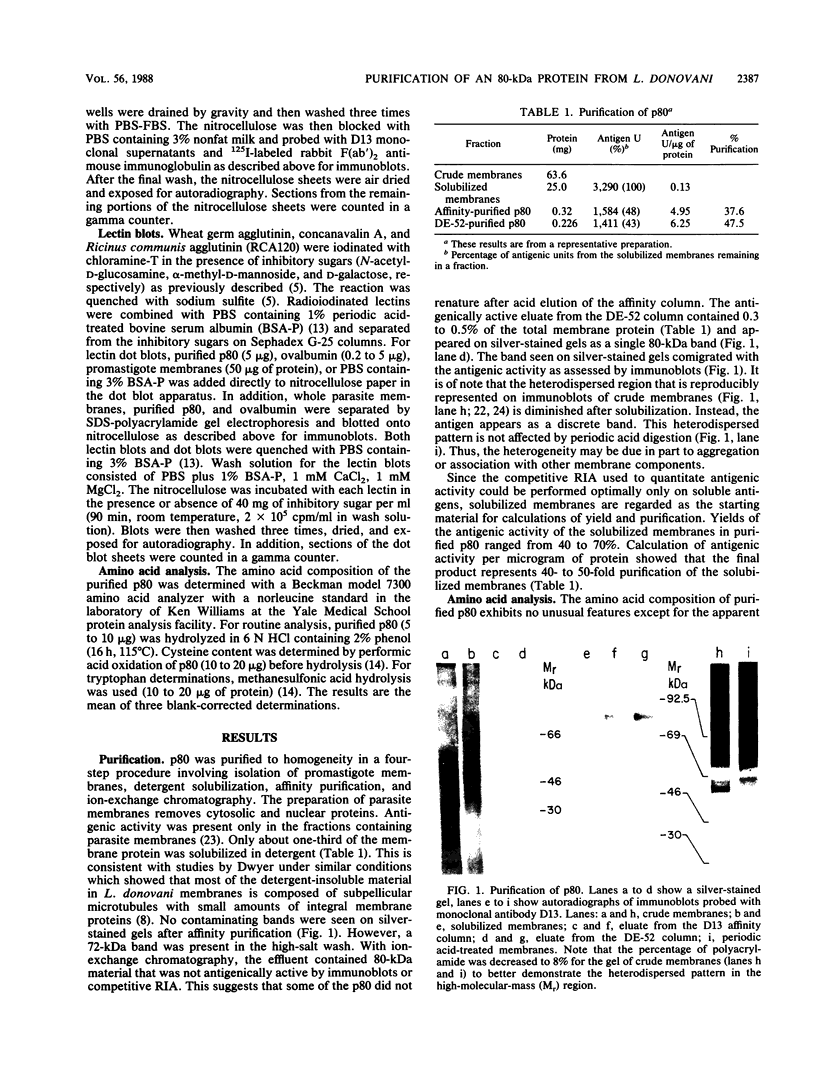
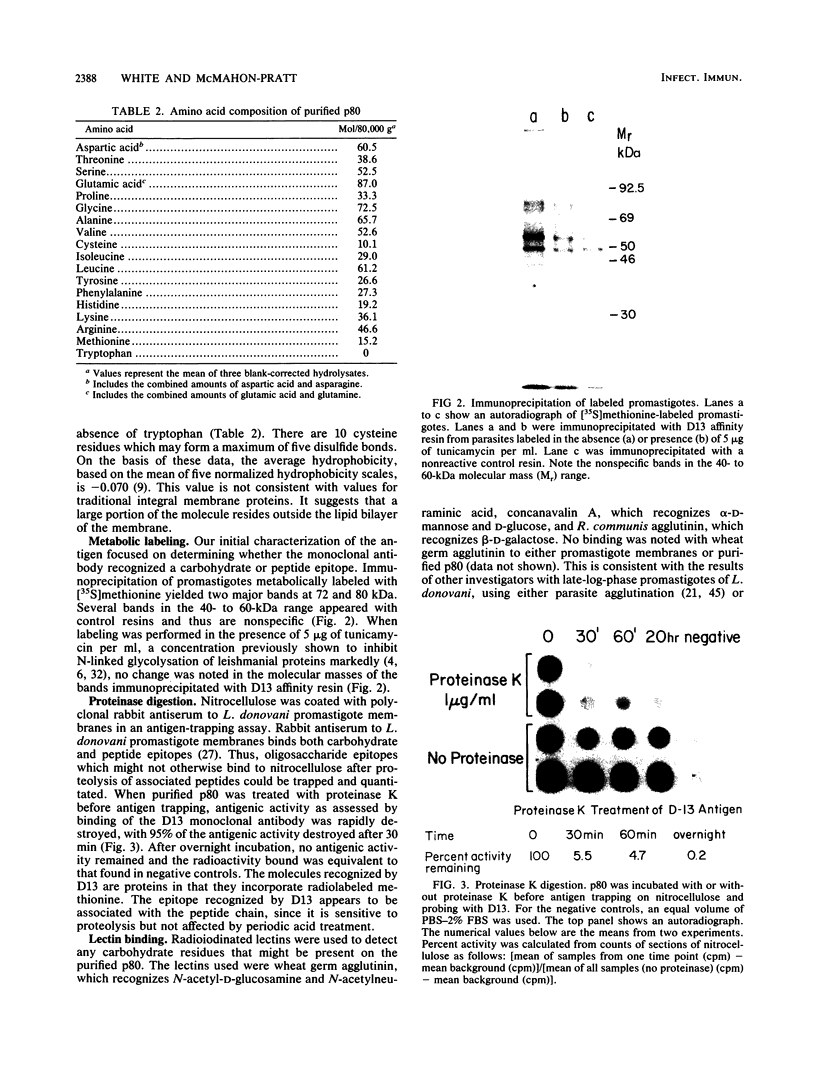
Visceral leishmaniasis is caused by the protozoan parasite Leishmania donovani. We previously described the development of 16 monoclonal antibodies specific for L. donovani. The epitope recognized by one of these monoclonal antibodies, D13, is present at high density on nearly all isolates of L. donovani and, along with two other monoclonal antibodies, has been used to develop a sensitive and specific competitive assay for serodiagnosis of visceral leishmaniasis. In this report, we characterize the antigens recognized by D13 by immunoprecipitation of [35S]methionine-labeled promastigotes as two proteins (apparent molecular mass, 72 and 80 kilodaltons). Pulse-chase studies showed no evidence of a precursor-product relationship for the two proteins. We purified the 80-kilodalton protein (p80) to homogeneity by detergent solubilization of promastigote membranes, immunoaffinity chromatography, and ion-exchange chromatography. The epitope on p80 recognized by D13 was completely destroyed by proteolysis but was not affected by periodic acid treatment. P80 did not bind to the radioiodinated lectins concanavalin A, wheat germ agglutinin, and Ricinus communis agglutinin. Its apparent molecular mass was not affected by tunicamycin. Thus, it does not appear to be glycosylated. This protein is highly immunogenic and may prove useful for immunoprophylaxis and serodiagnosis of visceral leishmaniasis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badaro R., Jones T. C., Carvalho E. M., Sampaio D., Reed S. G., Barral A., Teixeira R., Johnson W. D., Jr New perspectives on a subclinical form of visceral leishmaniasis. J Infect Dis. 1986 Dec;154(6):1003–1011. doi: 10.1093/infdis/154.6.1003. [DOI] [PubMed] [Google Scholar]
- Badaró R., Reed S. G., Barral A., Orge G., Jones T. C. Evaluation of the micro enzyme-linked immunosorbent assay (ELISA) for antibodies in American visceral leishmaniasis: antigen selection for detection of infection-specific responses. Am J Trop Med Hyg. 1986 Jan;35(1):72–78. doi: 10.4269/ajtmh.1986.35.72. [DOI] [PubMed] [Google Scholar]
- Badaró R., Reed S. G., Carvalho E. M. Immunofluorescent antibody test in American visceral leishmaniasis: sensitivity and specificity of different morphological forms of two Leishmania species. Am J Trop Med Hyg. 1983 May;32(3):480–484. doi: 10.4269/ajtmh.1983.32.480. [DOI] [PubMed] [Google Scholar]
- Bates P. A., Dwyer D. M. Biosynthesis and secretion of acid phosphatase by Leishmania donovani promastigotes. Mol Biochem Parasitol. 1987 Dec;26(3):289–296. doi: 10.1016/0166-6851(87)90081-8. [DOI] [PubMed] [Google Scholar]
- Burridge K. Direct identification of specific glycoproteins and antigens in sodium dodecyl sulfate gels. Methods Enzymol. 1978;50:54–64. doi: 10.1016/0076-6879(78)50007-4. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Inserra T. J., Kink J. A., Fong D., Chang K. P. Expression and size heterogeneity of a 63 kilodalton membrane glycoprotein during growth and transformation of Leishmania mexicana amazonensis. Mol Biochem Parasitol. 1986 Feb;18(2):197–210. doi: 10.1016/0166-6851(86)90038-1. [DOI] [PubMed] [Google Scholar]
- Colomer-Gould V., Glvao Quintao L., Keithly J., Nogueira N. A common major surface antigen on amastigotes and promastigotes of Leishmania species. J Exp Med. 1985 Sep 1;162(3):902–916. doi: 10.1084/jem.162.3.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D. Three-dimensional structure of membrane and surface proteins. Annu Rev Biochem. 1984;53:595–623. doi: 10.1146/annurev.bi.53.070184.003115. [DOI] [PubMed] [Google Scholar]
- Etges R. J., Bouvier J., Hoffman R., Bordier C. Evidence that the major surface proteins of three Leishmania species are structurally related. Mol Biochem Parasitol. 1985 Feb;14(2):141–149. doi: 10.1016/0166-6851(85)90033-7. [DOI] [PubMed] [Google Scholar]
- Etges R., Bouvier J., Bordier C. The major surface protein of Leishmania promastigotes is anchored in the membrane by a myristic acid-labeled phospholipid. EMBO J. 1986 Mar;5(3):597–601. doi: 10.1002/j.1460-2075.1986.tb04252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Guerrero M. L., Aguado J. M., Buzón L., Barros C., Montalbán C., Martín T., Bouza E. Visceral leishmaniasis in immunocompromised hosts. Am J Med. 1987 Dec;83(6):1098–1102. doi: 10.1016/0002-9343(87)90948-x. [DOI] [PubMed] [Google Scholar]
- Glass W. F., 2nd, Briggs R. C., Hnilica L. S. Use of lectins for detection of electrophoretically separated glycoproteins transferred onto nitrocellulose sheets. Anal Biochem. 1981 Jul 15;115(1):219–224. doi: 10.1016/0003-2697(81)90549-2. [DOI] [PubMed] [Google Scholar]
- Grimaldi G., Jr, David J. R., McMahon-Pratt D. Identification and distribution of New World Leishmania species characterized by serodeme analysis using monoclonal antibodies. Am J Trop Med Hyg. 1987 Mar;36(2):270–287. doi: 10.4269/ajtmh.1987.36.270. [DOI] [PubMed] [Google Scholar]
- Handman E., Greenblatt C. L., Goding J. W. An amphipathic sulphated glycoconjugate of Leishmania: characterization with monoclonal antibodies. EMBO J. 1984 Oct;3(10):2301–2306. doi: 10.1002/j.1460-2075.1984.tb02130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handman E., Mitchell G. F. Immunization with Leishmania receptor for macrophages protects mice against cutaneous leishmaniasis. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5910–5914. doi: 10.1073/pnas.82.17.5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath S., Chance M. L., Hommel M., Crampton J. M. Cloning of a gene encoding the immunodominant surface antigen of Leishmania donovani promastigotes. Mol Biochem Parasitol. 1987 Apr;23(3):211–222. doi: 10.1016/0166-6851(87)90028-4. [DOI] [PubMed] [Google Scholar]
- Ho M., Leeuwenburg J., Mbugua G., Wamachi A., Voller A. An enzyme-linked immunosorbent assay (ELISA) for field diagnosis of visceral leishmaniasis. Am J Trop Med Hyg. 1983 Sep;32(5):943–946. doi: 10.4269/ajtmh.1983.32.943. [DOI] [PubMed] [Google Scholar]
- Jacobson R. L., Slutzky G. M., Greenblatt C. L., Schnur L. F. Surface reaction of Leishmania. I. Lectin-mediated agglutination. Ann Trop Med Parasitol. 1982 Feb;76(1):45–52. [PubMed] [Google Scholar]
- Jaffe C. L., Bennett E., Grimaldi G., Jr, McMahon-Pratt D. Production and characterization of species-specific monoclonal antibodies against Leishmania donovani for immunodiagnosis. J Immunol. 1984 Jul;133(1):440–447. [PubMed] [Google Scholar]
- Jaffe C. L., McMahon-Pratt D. Serodiagnostic assay for visceral leishmaniasis employing monoclonal antibodies. Trans R Soc Trop Med Hyg. 1987;81(4):587–594. doi: 10.1016/0035-9203(87)90418-4. [DOI] [PubMed] [Google Scholar]
- Jaffe C. L., Zalis M. Purification of two Leishmania donovani membrane proteins recognized by sera from patients with visceral leishmaniasis. Mol Biochem Parasitol. 1988 Jan 1;27(1):53–62. doi: 10.1016/0166-6851(88)90024-2. [DOI] [PubMed] [Google Scholar]
- Kahl L. P., McMahon-Pratt D. Structural and antigenic characterization of a species- and promastigote-specific Leishmania mexicana amazonensis membrane protein. J Immunol. 1987 Mar 1;138(5):1587–1595. [PubMed] [Google Scholar]
- Kaneshiro E. S., Gottlieb M., Dwyer D. M. Cell surface origin of antigens shed by Leishmania donovani during growth in axenic culture. Infect Immun. 1982 Aug;37(2):558–567. doi: 10.1128/iai.37.2.558-567.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye P. M., Roberts M. B., Blackwell J. M. Analysing the immune response to L. donovani infection. Ann Inst Pasteur Immunol. 1987 Sep-Oct;138(5):762–768. doi: 10.1016/s0769-2625(87)80034-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepay D. A., Nogueira N., Cohn Z. Surface antigens of Leishmania donovani promastigotes. J Exp Med. 1983 May 1;157(5):1562–1572. doi: 10.1084/jem.157.5.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelace J. K., Gottlieb M. Effect of tunicamycin on the extracellular acid phosphatase of Leishmania donovani promastigotes. Mol Biochem Parasitol. 1987 Jan 2;22(1):19–28. doi: 10.1016/0166-6851(87)90065-x. [DOI] [PubMed] [Google Scholar]
- Parikh I., March S., Cuatercasas P. Topics in the methodology of substitution reactions with agarose. Methods Enzymol. 1974;34:77–102. doi: 10.1016/s0076-6879(74)34009-8. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Reed S. G., Badaro R., Lloyd R. M. Identification of specific and cross-reactive antigens of Leishmania donovani chagasi by human infection sera. J Immunol. 1987 Mar 1;138(5):1596–1601. [PubMed] [Google Scholar]
- Russell D. G., Alexander J. Effective immunization against cutaneous leishmaniasis with defined membrane antigens reconstituted into liposomes. J Immunol. 1988 Feb 15;140(4):1274–1279. [PubMed] [Google Scholar]
- Sacks D. L., Lal S. L., Shrivastava S. N., Blackwell J., Neva F. A. An analysis of T cell responsiveness in Indian kala-azar. J Immunol. 1987 Feb 1;138(3):908–913. [PubMed] [Google Scholar]
- Slutzky G. M., Londner M. V., Greenblatt C. L. Lipid and lipopolysaccharide-like antigens of Leishmania promastigotes. J Protozool. 1985 May;32(2):347–352. doi: 10.1111/j.1550-7408.1985.tb03064.x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turco S. J., Wilkerson M. A., Clawson D. R. Expression of an unusual acidic glycoconjugate in Leishmania donovani. J Biol Chem. 1984 Mar 25;259(6):3883–3889. [PubMed] [Google Scholar]
- Williams A. F. Assays for cellular antigens in the presence of detergents. Eur J Immunol. 1973 Oct;3(10):628–632. doi: 10.1002/eji.1830031007. [DOI] [PubMed] [Google Scholar]
- Wilson M. E., Pearson R. D. Stage-specific variations in lectin binding to Leishmania donovani. Infect Immun. 1984 Oct;46(1):128–134. doi: 10.1128/iai.46.1.128-134.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]