Abstract
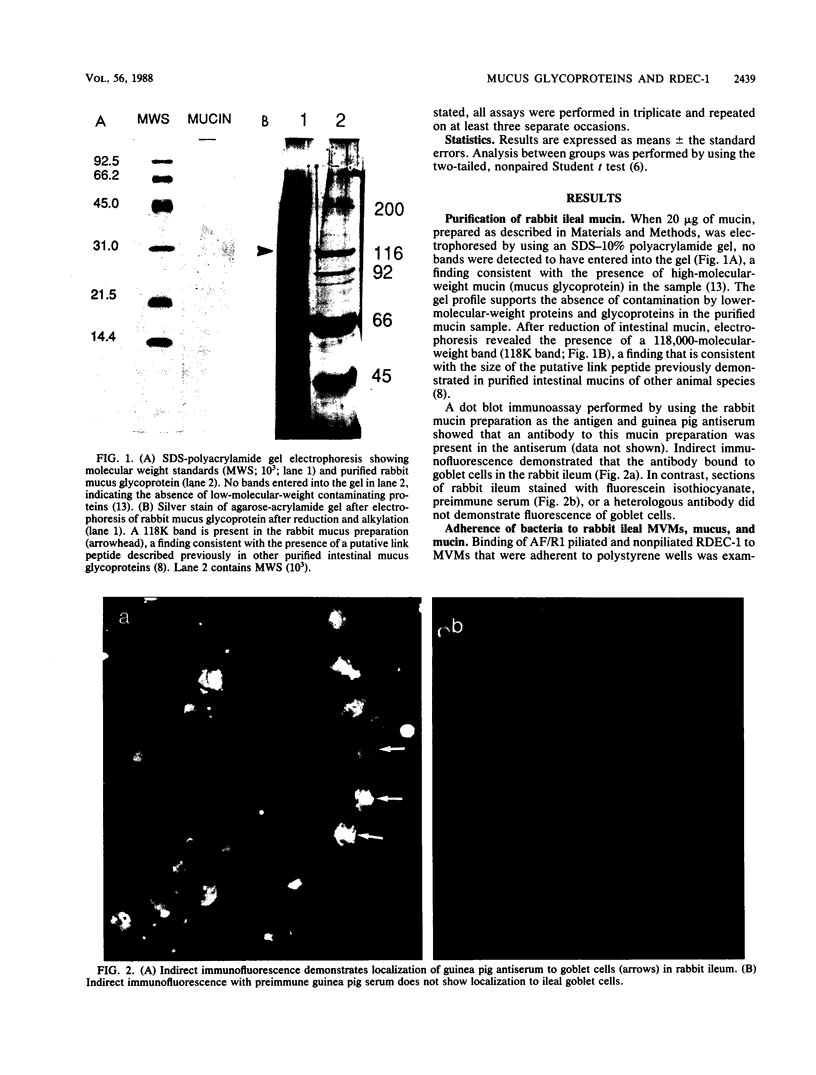
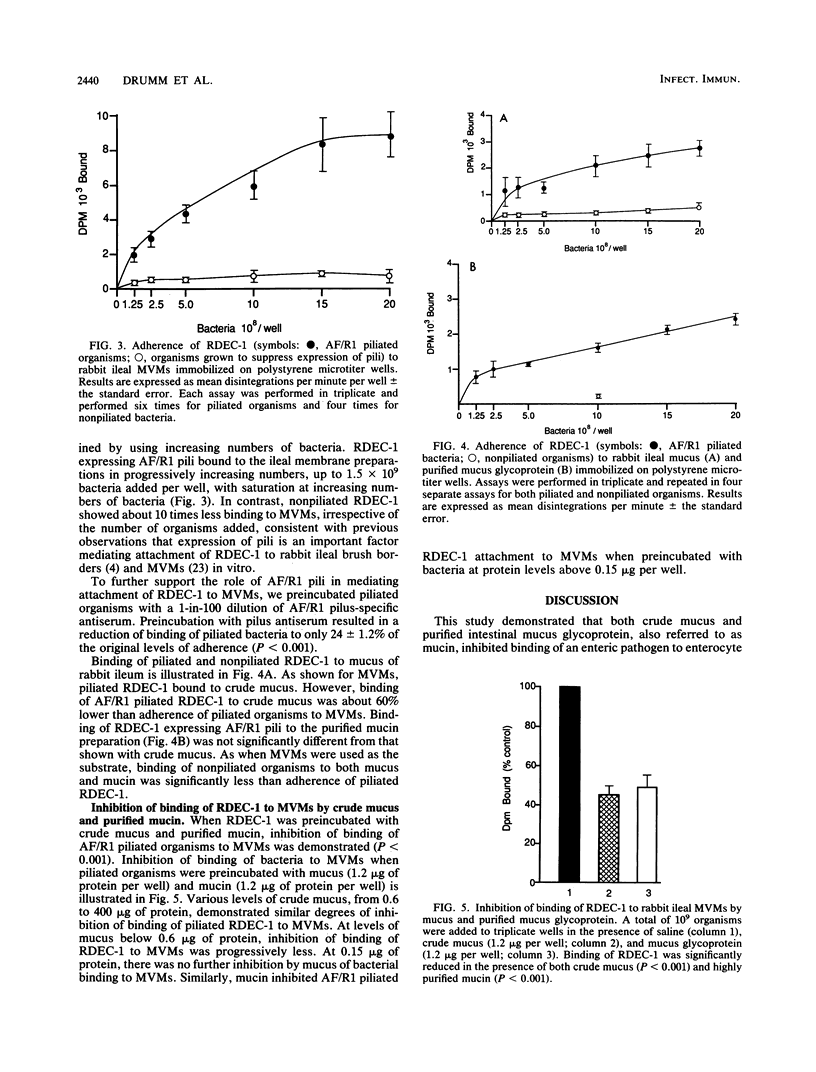
Intestinal mucus is postulated to play a role in preventing colonization of the gastrointestinal tract by microbial pathogens. To evaluate the ability of both crude mucus and purified mucin, a glycoprotein of goblet cell origin, to inhibit mucosal adherence of enteric pathogens, we examined whether mucus and mucin derived from rabbit ileum interact with the rabbit enteropathogen Escherichia coli RDEC-1. We examined the manner in which mucus and mucin inhibited adherence of bacteria to rabbit ileal microvillus membranes (MVMs) in vitro. The purity of the mucin preparation was demonstrated by polyacrylamide gel electrophoresis before and after reduction and by showing that an antiserum raised to the mucin localized to goblet cells in rabbit intestine. Using radioactive labeling of bacteria, we quantitated attachment of RDEC-1 to MVMs, mucus, and mucin that had been immobilized on polystyrene microtiter wells. Binding of RDEC-1 to MVMs was also determined after preincubation of organisms with crude ileal mucus and purified mucin. RDEC-1 bound to both crude mucus and purified mucin when they expressed lectinlike adhesions, previously designated attachment factor rabbit 1 pili. Adherence of piliated RDEC-1 to MVMs, mucus, and mucin was significantly greater than when the bacteria were nonpiliated. Binding of piliated RDEC-1 to MVMs was decreased by preincubation of bacteria with both crude mucus (45.6 +/- 4.2% of control) and purified mucin (50.2 +/- 5.8%). These data indicate that the E. coli enteropathogen RDEC-1 can bind to purified glycoproteins of goblet cell origin and that adherence of these bacteria to mucin is mediated by expression of pili. The findings also support a role for intestinal mucus and its principal organic constituent, mucin, in preventing adherence of a known E. coli enteric pathogen to apical MVMs of enterocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen A., Hutton D. A., Pearson J. P., Sellers L. A. Mucus glycoprotein structure, gel formation and gastrointestinal mucus function. Ciba Found Symp. 1984;109:137–156. doi: 10.1002/9780470720905.ch10. [DOI] [PubMed] [Google Scholar]
- Beachey E. H. Bacterial adherence: adhesin-receptor interactions mediating the attachment of bacteria to mucosal surface. J Infect Dis. 1981 Mar;143(3):325–345. doi: 10.1093/infdis/143.3.325. [DOI] [PubMed] [Google Scholar]
- Cantey J. R., Blake R. K. Diarrhea due to Escherichia coli in the rabbit: a novel mechanism. J Infect Dis. 1977 Mar;135(3):454–462. doi: 10.1093/infdis/135.3.454. [DOI] [PubMed] [Google Scholar]
- Cheney C. P., Formal S. B., Schad P. A., Boedeker E. C. Genetic transfer of a mucosal adherence factor (R1) from an enteropathogenic Escherichia coli strain into a Shigella flexneri strain and the phenotypic suppression of this adherence factor. J Infect Dis. 1983 Apr;147(4):711–723. doi: 10.1093/infdis/147.4.711. [DOI] [PubMed] [Google Scholar]
- Cheney C. P., Schad P. A., Formal S. B., Boedeker E. C. Species specificity of in vitro Escherichia coli adherence to host intestinal cell membranes and its correlation with in vivo colonization and infectivity. Infect Immun. 1980 Jun;28(3):1019–1027. doi: 10.1128/iai.28.3.1019-1027.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahim R. E., Specian R. D., Forstner G. G., Forstner J. F. Characterization and localization of the putative 'link' component in rat small-intestinal mucin. Biochem J. 1987 May 1;243(3):631–640. doi: 10.1042/bj2430631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forstner J. F. Intestinal mucins in health and disease. Digestion. 1978;17(3):234–263. doi: 10.1159/000198115. [DOI] [PubMed] [Google Scholar]
- Kessler M., Acuto O., Storelli C., Murer H., Müller M., Semenza G. A modified procedure for the rapid preparation of efficiently transporting vesicles from small intestinal brush border membranes. Their use in investigating some properties of D-glucose and choline transport systems. Biochim Biophys Acta. 1978 Jan 4;506(1):136–154. doi: 10.1016/0005-2736(78)90440-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laboisse C. L. Structure of gastrointestinal mucins: searching for the Rosetta stone. Biochimie. 1986 May;68(5):611–617. doi: 10.1016/s0300-9084(86)80155-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mantle M., Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings]. Biochem Soc Trans. 1978;6(3):607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- Mantle M., Allen A. Isolation and characterization of the native glycoprotein from pig small-intestinal mucus. Biochem J. 1981 Apr 1;195(1):267–275. doi: 10.1042/bj1950267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouricout M. A., Julien R. A. Pilus-mediated binding of bovine enterotoxigenic Escherichia coli to calf small intestinal mucins. Infect Immun. 1987 May;55(5):1216–1223. doi: 10.1128/iai.55.5.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi R., Forstner G. G., Forstner J. F. Radioimmunoassay of human intestinal goblet cell mucin. Investigation of mucus from different organs and species. J Clin Invest. 1979 Nov;64(5):1149–1156. doi: 10.1172/JCI109568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman P. M., Boedeker E. C. Pilus-mediated interactions of the Escherichia coli strain RDEC-1 with mucosal glycoproteins in the small intestine of rabbits. Gastroenterology. 1987 Oct;93(4):734–743. doi: 10.1016/0016-5085(87)90435-5. [DOI] [PubMed] [Google Scholar]
- Sherman P. M., Boedeker E. C. Regional differences in attachment of enteroadherent Escherichia coli strain RDEC-1 to rabbit intestine: luminal colonization but lack of mucosal adherence in jejunal self-filling blind loops. J Pediatr Gastroenterol Nutr. 1987 May-Jun;6(3):439–444. doi: 10.1097/00005176-198705000-00022. [DOI] [PubMed] [Google Scholar]
- Sherman P. M., Houston W. L., Boedeker E. C. Functional heterogeneity of intestinal Escherichia coli strains expressing type 1 somatic pili (fimbriae): assessment of bacterial adherence to intestinal membranes and surface hydrophobicity. Infect Immun. 1985 Sep;49(3):797–804. doi: 10.1128/iai.49.3.797-804.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi A., Inman L. R., O'Hanley P. D., Cantey J. R., Lushbaugh W. B. Scanning and transmission electron microscopic study of Escherichia coli O15 (RDEC-1) enteric infection in rabbits. Infect Immun. 1978 Feb;19(2):686–694. doi: 10.1128/iai.19.2.686-694.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Ulshen M. H., Rollo J. L. Pathogenesis of escherichia coli gastroenteritis in man--another mechanism. N Engl J Med. 1980 Jan 10;302(2):99–101. doi: 10.1056/NEJM198001103020207. [DOI] [PubMed] [Google Scholar]





