Abstract
An animal model for evaluating the potency of Mycoplasma pneumoniae vaccines was developed with hamsters. Factors that influence hamster infection by M. pneumoniae were defined, and parameters for assessment of intensity of pulmonary disease were established. Colonization of hamster lungs was determined by culture, and intensity of lung disease was assessed histopathologically and expressed numerically as a lung pathological score. Intratracheal inoculation of the challenge was superior to the intranasal or aerosol route for inducing a consistent degree of lung disease. A challenge dose of 10(6) CFU inoculated intratracheally produced lung colonization and significant reproducible lung pathological scores in essentially all unvaccinated animals. The peak of infection, as determined by these criteria, was at about 2 weeks after challenge. Animals over 6 weeks of age were preferable for the test, since younger animals exhibited a lower lung pathological score even though they showed the same degree of lung colonization. The hamster assay developed provides a dependable experimental system for testing the protective potency of M. pneumoniae vaccines.
Full text
PDF

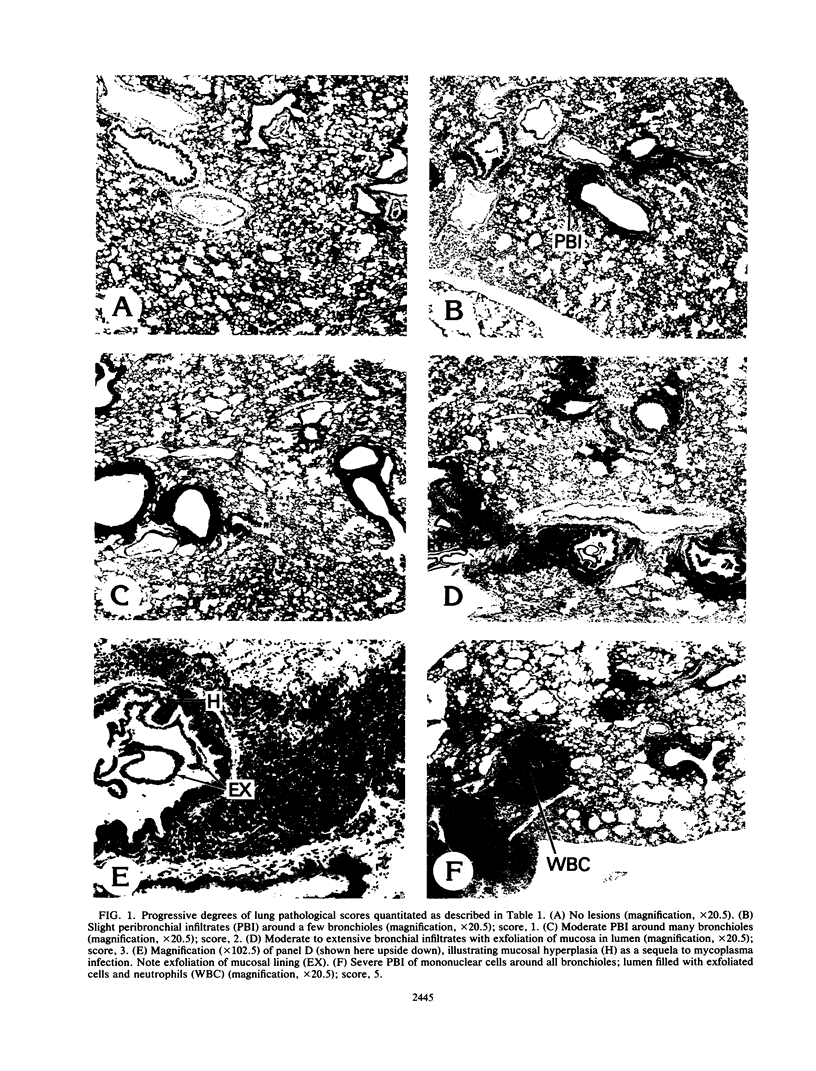

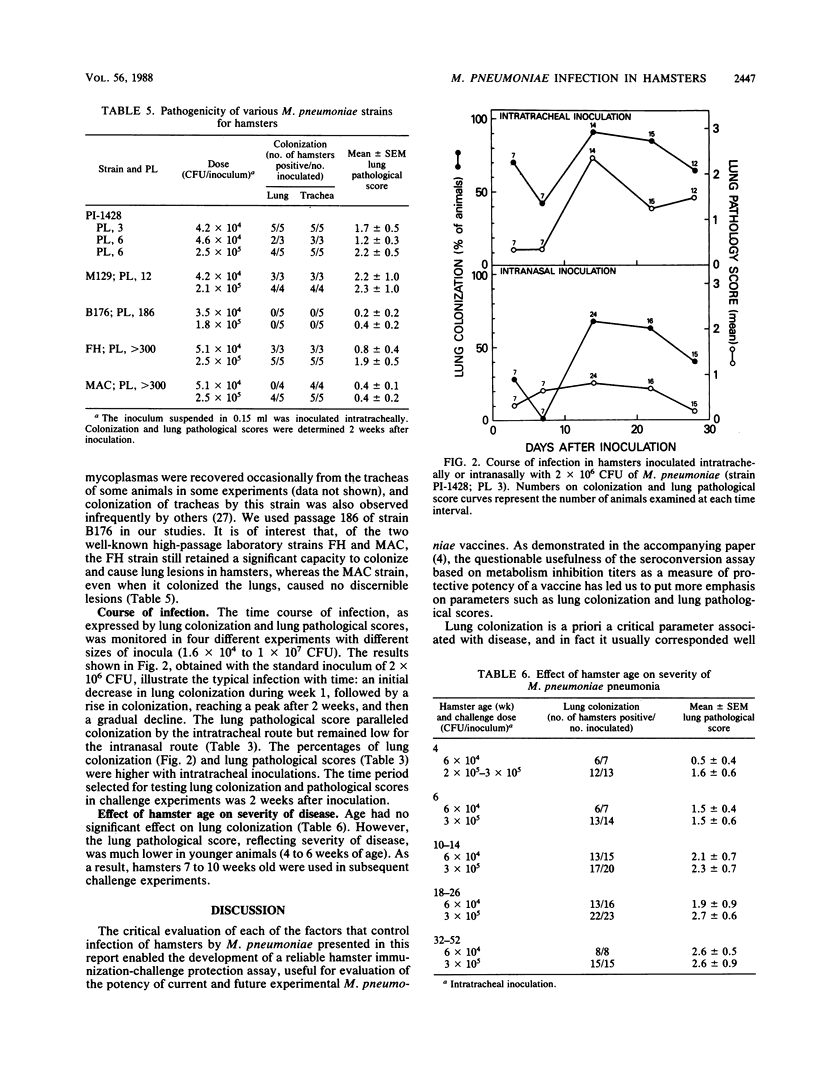


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barile M. F., Chandler D. K., Yoshida H., Grabowski M. W., Harasawa R., Ahmed O. A. Hamster challenge potency assay for evaluation of Mycoplasma pneumoniae vaccines. Isr J Med Sci. 1981 Jul;17(7):682–686. [PubMed] [Google Scholar]
- Barile M. F., Chandler D. K., Yoshida H., Grabowski M. W., Razin S. Hamster challenge potency assay for evaluation of Mycoplasma pneumoniae vaccines. Infect Immun. 1988 Sep;56(9):2450–2457. doi: 10.1128/iai.56.9.2450-2457.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner H., Feldner J., Bredt W. Effect of monoclonal antibodies to the attachment-tip on experimental Mycoplasma pneumoniae infection of hamsters. A preliminary report. Isr J Med Sci. 1984 Sep;20(9):878–881. [PubMed] [Google Scholar]
- Brunner H., Prescott B., Greenberg H., James W. D., Horswood R. L., Chanock R. M. Unexpectedly high frequency of antibody to Mycoplasma pneumoniae in human sera as measured by sensitive techniques. J Infect Dis. 1977 Apr;135(4):524–530. doi: 10.1093/infdis/135.4.524. [DOI] [PubMed] [Google Scholar]
- Brunner H. Protective efficacy of Mycoplasma pneumoniae polysaccharides. Isr J Med Sci. 1981 Jul;17(7):678–681. [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Ciliostatic, hemagglutinating, and proteolytic activities in a cell extract of Mycoplasma pneumoniae. Infect Immun. 1980 Sep;29(3):1111–1116. doi: 10.1128/iai.29.3.1111-1116.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Barile M. F. Mycoplasma pneumoniae attachment to WiDr cell cultures: competitive inhibition assays. Yale J Biol Med. 1983 Sep-Dec;56(5-6):679–683. [PMC free article] [PubMed] [Google Scholar]
- Chandler D. K., Grabowski M. W., Rabson A. S., Barile M. F. Further studies on the Mycoplasma pneumoniae extract: ciliostatic and cell recruitment activities. Isr J Med Sci. 1987 Jun;23(6):580–584. [PubMed] [Google Scholar]
- Clyde W. A., Jr Immunopathology of experimental Mycoplasma pneumoniae disease. Infect Immun. 1971 Dec;4(6):757–763. doi: 10.1128/iai.4.6.757-763.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clyde W. A., Jr Mycoplasma pneumoniae respiratory disease symposium: summation and significance. Yale J Biol Med. 1983 Sep-Dec;56(5-6):523–527. [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr, Denny F. W. Biologic effects of Mycoplasma pneumoniae and other mycoplasmas from man on hamster tracheal organ culture. Proc Soc Exp Biol Med. 1969 Dec;132(3):1153–1158. doi: 10.3181/00379727-132-34385. [DOI] [PubMed] [Google Scholar]
- DAJANI A. S., CLYDE W. A., Jr, DENNY F. W. EXPERIMENTAL INFECTION WITH MYCOPLASMA PNEUMONIAE (EATON'S AGENT). J Exp Med. 1965 Jun 1;121:1071–1086. doi: 10.1084/jem.121.6.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny F. W., Clyde W. A., Jr, Glezen W. P. Mycoplasma pneumoniae disease: clinical spectrum, pathophysiology, epidemiology, and control. J Infect Dis. 1971 Jan;123(1):74–92. doi: 10.1093/infdis/123.1.74. [DOI] [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A., Jr, Bienenstock J. Immunoglobulin-containing cells in lungs of hamsters infected with Mycoplasma pneumoniae. J Immunol. 1972 May;108(5):1400–1408. [PubMed] [Google Scholar]
- Fernald G. W., Clyde W. A. Protective Effect of Vaccines in Experimental Mycoplasma pneumoniae Disease. Infect Immun. 1970 Jun;1(6):559–565. doi: 10.1128/iai.1.6.559-565.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M. J., Telfer Brunton W. A., Millar J., Stewart C., Critchley J. A. Mycoplasma pneumonia: failure of erythromycin therapy. Scott Med J. 1980 Apr;25(2):126–128. doi: 10.1177/003693308002500208. [DOI] [PubMed] [Google Scholar]
- Hayatsu E. Acquired immunity to Mycoplasma pneumoniae. Pneumonia in hamsters. Microbiol Immunol. 1978;22(4):181–195. doi: 10.1111/j.1348-0421.1978.tb00362.x. [DOI] [PubMed] [Google Scholar]
- Kaklamanis E., Thomas L., Stavropoulos K., Borman I., Boshwitz C. Mycoplasmacidal action of normal tissue extracts. Nature. 1969 Mar 1;221(5183):860–862. doi: 10.1038/221860b0. [DOI] [PubMed] [Google Scholar]
- Komaroff A. L., Aronson M. D., Pass T. M., Ervin C. T., Branch W. T., Jr, Schachter J. Serologic evidence of chlamydial and mycoplasmal pharyngitis in adults. Science. 1983 Nov 25;222(4626):927–929. doi: 10.1126/science.6415813. [DOI] [PubMed] [Google Scholar]
- Larson E. W., Young H. W., Walker J. S. Aerosol evaluations of the DeVilbiss No. 40 and Vaponefrin nebulizers. Appl Environ Microbiol. 1976 Jan;31(1):150–151. doi: 10.1128/aem.31.1.150-151.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman R. P., Clyde W. A., Jr, Denny F. W. Characteristics of virulent, attenuated, and avirulent Mycoplasma pneumoniae strains. J Bacteriol. 1969 Nov;100(2):1037–1043. doi: 10.1128/jb.100.2.1037-1043.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg P., White R. J., Fuld S. L., Gutekunst R. R., Chanock R. M., Senterfit L. B. Ecology of Mycoplasma pneumoniae infections in marine recruits at Parris Island, South Carolina. Am J Epidemiol. 1969 Jan;89(1):62–73. doi: 10.1093/oxfordjournals.aje.a120916. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Purcell R. H., Wong D. C., Chanock R. M. A colour test for the measurement of antibody to certain mycoplasma species based upon the inhibition of acid production. J Hyg (Lond) 1966 Mar;64(1):91–104. doi: 10.1017/s0022172400040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu A. C., Foy H. M., Cartwright F. D., Kenny G. E. The principal protein antigens of isolates of Mycoplasma pneumoniae as measured by levels of immunoglobulin G in human serum are stable in strains collected over a 10-year period. Infect Immun. 1987 Aug;55(8):1830–1836. doi: 10.1128/iai.55.8.1830-1836.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev D., Halachmi D., Kenny G. E., Razin S. Distinction of species and strains of mycoplasmas (mollicutes) by genomic DNA fingerprints with an rRNA gene probe. J Clin Microbiol. 1988 Jun;26(6):1198–1201. doi: 10.1128/jcm.26.6.1198-1201.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]