Abstract
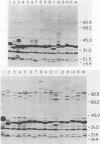
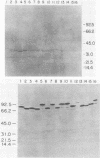
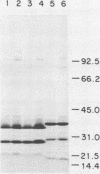
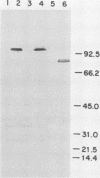
An 84-kilodalton outer membrane protein was expressed when Pasteurella multocida, somatic serotype 3, was grown in brain-heart infusion broth containing the iron chelator dipyridyl but not in brain-heart infusion broth alone. Antigenically related outer membrane proteins of various molecular masses were also expressed by P. multocida strains belonging to all of the other 15 somatic serotypes (somatic serotype 12 being the possible exception) as well as by isolates expressing somatic antigens representative of multiple somatic serotypes when grown under the same conditions of iron deprivation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagg A., Neilands J. B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987 Dec;51(4):509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierer B. W., Derieux W. T. Immunologic response of turkeys to an avirulent Pasteurella multocida vaccine in the drinking water. Poult Sci. 1972 Mar;51(2):408–416. doi: 10.3382/ps.0510408. [DOI] [PubMed] [Google Scholar]
- Bindereif A., Braun V., Hantke K. The cloacin receptor of ColV-bearing Escherichia coli is part of the Fe3+-aerobactin transport system. J Bacteriol. 1982 Jun;150(3):1472–1475. doi: 10.1128/jb.150.3.1472-1475.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolin C. A., Jensen A. E. Passive immunization with antibodies against iron-regulated outer membrane proteins protects turkeys from Escherichia coli septicemia. Infect Immun. 1987 May;55(5):1239–1242. doi: 10.1128/iai.55.5.1239-1242.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chart H., Buck M., Stevenson P., Griffiths E. Iron regulated outer membrane proteins of Escherichia coli: variations in expression due to the chelator used to restrict the availability of iron. J Gen Microbiol. 1986 May;132(5):1373–1378. doi: 10.1099/00221287-132-5-1373. [DOI] [PubMed] [Google Scholar]
- Chart H., Griffiths E. Antigenic and molecular homology of the ferric enterobactin receptor protein of Escherichia coli. J Gen Microbiol. 1985 Jun;131(6):1503–1509. doi: 10.1099/00221287-131-6-1503. [DOI] [PubMed] [Google Scholar]
- Cox C. D. Iron uptake with ferripyochelin and ferric citrate by Pseudomonas aeruginosa. J Bacteriol. 1980 May;142(2):581–587. doi: 10.1128/jb.142.2.581-587.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis P. E. Observations on avian pasteurellosis in Britain. Vet Rec. 1979 May 26;104(21):471–474. doi: 10.1136/vr.104.21.471. [DOI] [PubMed] [Google Scholar]
- Derieux W. T. Response of young chickens and turkeys to virulent and avirulent Pasteurella multocida administered by various routes. Avian Dis. 1978 Jan-Mar;22(1):131–139. [PubMed] [Google Scholar]
- Filip C., Fletcher G., Wulff J. L., Earhart C. F. Solubilization of the cytoplasmic membrane of Escherichia coli by the ionic detergent sodium-lauryl sarcosinate. J Bacteriol. 1973 Sep;115(3):717–722. doi: 10.1128/jb.115.3.717-722.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiss E. H., Stanley-Samuelson P., Neilands J. B. Properties and proteolysis of ferric enterobactin outer membrane receptor in Escherichia coli K12. Biochemistry. 1982 Aug 31;21(18):4517–4522. doi: 10.1021/bi00261a050. [DOI] [PubMed] [Google Scholar]
- Heddleston K. L., Gallagher J. E., Rebers P. A. Fowl cholera: immune response in turkeys. Avian Dis. 1970 Nov;14(4):626–635. [PubMed] [Google Scholar]
- Heddleston K. L., Rebers P. A. Fowl cholera: cross-immunity induced in turkeys with formalin-killed in-vivo-propagated Pasteurella. Avian Dis. 1972 May-Jun;16(3):578–586. [PubMed] [Google Scholar]
- Hofacre C. L., Glisson J. R. A serotypic survey of Pasteurella multocida isolated from poultry. Avian Dis. 1986 Jul-Sep;30(3):632–633. [PubMed] [Google Scholar]
- Hu S. P., Felice L. J., Sivanandan V., Maheswaran S. K. Siderophore production by Pasteurella multocida. Infect Immun. 1986 Dec;54(3):804–810. doi: 10.1128/iai.54.3.804-810.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunkapiller M. W., Lujan E., Ostrander F., Hood L. E. Isolation of microgram quantities of proteins from polyacrylamide gels for amino acid sequence analysis. Methods Enzymol. 1983;91:227–236. doi: 10.1016/s0076-6879(83)91019-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial envelope proteins related to iron. Annu Rev Microbiol. 1982;36:285–309. doi: 10.1146/annurev.mi.36.100182.001441. [DOI] [PubMed] [Google Scholar]
- Neilands J. B. Microbial iron compounds. Annu Rev Biochem. 1981;50:715–731. doi: 10.1146/annurev.bi.50.070181.003435. [DOI] [PubMed] [Google Scholar]
- Perry R. D., San Clemente C. L. Siderophore synthesis in Klebsiella pneumoniae and Shigella sonnei during iron deficiency. J Bacteriol. 1979 Dec;140(3):1129–1132. doi: 10.1128/jb.140.3.1129-1132.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimler R. B. Cross-protection factor(s) of Pasteurella multocida: passive immunization of turkeys against fowl cholera caused by different serotypes. Avian Dis. 1987 Oct-Dec;31(4):884–887. [PubMed] [Google Scholar]
- Rimler R. B., Rebers P. A., Rhoades K. R. Fowl cholera: cross-protection induced by Pasteurella multocida separated from infected turkey blood. Avian Dis. 1979 Jul-Sep;23(3):730–741. [PubMed] [Google Scholar]
- Schnaitman C. A. Examination of the protein composition of the cell envelope of Escherichia coli by polyacrylamide gel electrophoresis. J Bacteriol. 1970 Nov;104(2):882–889. doi: 10.1128/jb.104.2.882-889.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigel S. P., Payne S. M. Effect of iron limitation on growth, siderophore production, and expression of outer membrane proteins of Vibrio cholerae. J Bacteriol. 1982 Apr;150(1):148–155. doi: 10.1128/jb.150.1.148-155.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Characterization of antibody to the ferripyochelin-binding protein of Pseudomonas aeruginosa. Infect Immun. 1986 Mar;51(3):896–900. doi: 10.1128/iai.51.3.896-900.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Woods D. E. Demonstration of an iron-siderophore-binding protein in the outer membrane of Pseudomonas aeruginosa. Infect Immun. 1983 May;40(2):665–669. doi: 10.1128/iai.40.2.665-669.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]