Abstract
Although manipulation of the endoplasmic reticulum (ER) folding environment in the yeast Saccharomyces cerevisiae has been shown to increase the secretory productivity of recombinant proteins, the cellular interactions and processes of native enzymes and chaperones such as protein disulfide isomerase (PDI) are still unclear. Previously, we reported that overexpression of the ER chaperone PDI enabled up to a three-fold increase in secretion levels of the Pyrococcus furiosus β-glucosidase in the yeast S. cerevisiae. This result was surprising since β-glucosidase contains only one cysteine per monomer and no disulfide bonds. Two possible mechanisms were proposed: PDI either forms a transient disulfide bond with the lone cysteine residue of the nascent β-glucosidase during the folding and assembly process, or it acts as a chaperone to aid in proper folding. To discern between the two mechanisms, the single cysteine residue was mutated to serine, and the secretion of the two protein variants was determined. The serine mutant still showed increased secretion in vivo when PDI levels were elevated. When the folding bottleneck is removed by increasing expression temperatures to 37°C rather than 30°C, PDI no longer has an improvement on secretion. These results suggest that, unexpectedly, PDI acts in a chaperone-like capacity or possibly cooperates with the cell's folding or degradation mechanisms regardless of whether the protein is redox-active.
Keywords: Archaea, hyperthermophile, Saccharomyces cerevisiae, protein disulfide isomerase, β-glucosidase, Pyrococcus furiosus, CelB, chaperones
Introduction
Proteins isolated from organisms thriving under extreme conditions (extremophiles) are potentially ideal biocatalysts for industrial reactions where proteins from mesophilic organisms (surviving around room temperature, atmospheric pressure, and neutral pH or low salinities) fail due to unfavorable process conditions and storage limitations 1. However, obtaining large quantities of extremophilic enzymes is often difficult; cultivation of these organisms requires conditions outside the capabilities of most laboratory equipment, and even with specialized fermentors, the biomass yield from the native organisms is often low 2. Recombinant expression in a mesophilic host is an attractive alternative to overcoming this limitation, and in addition, the recombinantly produced protein can be tagged for secretion into the supernatant to simplify purification. Often, however, overexpression of a foreign protein can saturate the cellular machinery of a non-native host, limiting the protein yield that can be recovered from recombinant expression systems; one strategy that has successfully increased secretory productivity in recombinant systems is manipulation of the ER folding environment 3.
Previously, a recombinant expression system of the β-glucosidase from Pyrococcus furiosus, a hyperthermophilic organism with an optimum growth temperature around 100°C 4, in the yeast Saccharomyces cerevisiae was developed. While high yields of secreted protein up to 12 mg/L were achieved 5, a secretion bottleneck exists in the endoplasmic reticulum (ER), the cellular compartment in which secretory proteins are folded, processed, and assembled 6. In this system, elevated levels of protein disulfide isomerase (PDI) increased secretion, while high levels of BiP, also known as Kar2p in yeast, decreased secretion 6, 7.
How does PDI improve the expression of β-glucosidase? The role of PDI is traditionally understood to be an oxidizing/reducing/isomerizing agent to aid in correctly forming folds by shuffling incorrectly formed disulfide bonds 8, but in P. furiosus β-glucosidase there is only one cysteine residue. This protein is active in a stable tetrameric form where the cysteine residues in each subunit are separated by 38 Å or 67 Å (Figure 1a) (S.J.J. Brouns, T. Kaper, W.M. de Vos and J. van der Oost, unpublished data, used with permission). At these large distances, no intramolecular disulfide bonds are possible in the final structure. Therefore two possible roles for increased secretion at higher PDI levels were postulated 6. The first is that PDI forms a transient disulfide bond to nascent β-glucosidase, therefore limiting folding conformations or segregating the protein in its folding environment. Another possible but less understood role for PDI is that it acts in vivo as a folding chaperone, independent of its well-known isomerase function. While the chaperone activity of PDI is poorly understood, chaperone and anti-chaperone activity has been clearly demonstrated in folding other proteins 9, 10. All of these proteins, however, contained at least one cysteine residue, so it remains unclear whether the chaperone ability of PDI is recruited by cysteine residues in the unfolded β-glucosidase protein.
Figure 1.


a) Model of P. furiosus β-glucosidase tetramer based on 3.3Å X-ray diffraction data (S.J.J. Brouns, T. Kaper, W.M. de Vos and J. van der Oost, unpublished data, used with permission). Subunits are depicted in Cα trace presentation. The single cysteine residue in each monomer is highlighted in ball and stick presentation. Distance between cysteine residues in intramolecular subunits is 67 Å or 38 Å, as illustrated, too far apart to contain a disulfide in the folded structure. b) Native PAGE shows similar predominantly tetrameric structure between wild-type (Lane 1) and C75S (Lane 2) β-glucosidase.
Here we explored how PDI aids in folding and secretion of this β-glucosidase by mutating the single cysteine residue (C75) to a serine. Because the redox-activity of PDI itself is essential in S. cerevisiae8, 11, improvements in the secretion of β-glucosidase when PDI levels are increased were examined by removing the ability of β-glucosidase to interact with PDI through formation of a disulfide bond rather than introducing mutations into PDI itself. While we cannot definitively conclude whether the PDI is acting as a chaperone and directly interacting with this protein during folding, or if it is indirectly aiding the folding, secretion, or degradation machinery of the cell, we show an unfolded protein does not have to be redox active for PDI to facilitate an increase in secretion. While this result was unexpected, by further understanding the process of expressing recombinant proteins in S. cerevisiae, it will be possible to adapt cellular conditions to aid in the production of useful biocatalysts and other therapeutic proteins that are complex and require the assistance of chaperones and foldases.
Materials and Methods
Plasmids
The single copy expression plasmid pRS314-β was constructed from a secretion-modified full-length β-glucosidase with an N-terminal synthetic pre-pro secretion signal sequence and a 10-amino-acid C-terminal c-myc detection tag under the control of a GAL1-10 promoter and the plasmid pRS314 (ATCC) containing the TRP1 marker as reported previously 5. Site-directed mutagenesis was performed using the QuikChange Site-Directed Mutagenesis kit (Stratagene) according to manufacturer instructions using plasmid pRS314-β to create pRS314-βC75S containing the C75S mutation. The identity of the mutation was verified by complete sequencing of the gene (University of Pennsylvania Sequencing Center). pRS314-βC75S was digested with Kpn I and Sac I to give the GAL1-10 to alpha terminator expression cassette. The pITy4-β integrating plasmid 5 was cut using the Kpn I and Sac I sites, and the expression cassette containing the C75S mutation was ligated to form pITy4-βC75S.
Cell Strains
The S. cerevisiae yeast parental strain BJ5464 (MAT α ura3-52 trp1 leu2Δl hisΔ200 pep4::HIS3 prb1Δ1.6R can1 GAL), obtained from ATCC, served as the basis for all yeast constructs in this work. High levels of wild-type (wt) β-glucosidase were obtained by expression in JSIY017, created previously by transforming BJ5464 with the pITy4-β integrating plasmid by electroporation and subsequent screening for a high yield strain by resistance to G418 5. To produce high levels of C75S for purification and further studies, strain SPIY01 was created from BJ5464 transformed with pITy4-βC75S by electroporation and selected by plating on YPD containing 850μg/mL G418 12. The hPDI strain was created by transforming BJ5464 with the integrating plasmid pITYGAPDH-PDI containing the PDI1 gene and the GAPDH promoter 13. Both BJ5464 and hPDI strains were transformed with pRS314-β and pRS314-βC75S by electroporation to yield the strains BJ wt, hPDI wt, BJ C75S, and hPDI C75S.
Media and Culture Growth Conditions
Three colonies each of BJ wt, hPDI wt, BJ C75S, and hPDI C75S, were selected on the basis of growth on SD-2xSCAA dextrose media lacking tryptophan supplements 14, re-streaked, and grown twice to ensure a single plasmid-containing colony was isolated. Cultures were grown to saturation in SD-2xSCAA dextrose liquid growth media minus tryptophan supplements in 5 mL culture tubes at 30°C and 275 rpm. After 48 h, to switch to galactose expression media, 5 OD600 equivalents were removed and isolated by centrifugation at 2000g for 10 min. Supernatants were decanted, and the cell pellets were resuspended in 5 mL SG-2xSCAA galactose liquid expression media minus tryptophan supplements. The same procedure was followed for scale up to 25 mL culture flasks, with the exception that BSA was added at 1 mg/mL to the SD-2xSCAA minus tryptophan supplements to inhibit binding of secreted protein to the flasks. BSA was not added to the SG-2xSCAA minus tryptophan expression media because it interfered with Western blot analysis as BSA migrated at approximately the same molecular weight as the β-glucosidase monomers. The same culture flasks were used for growth and expression after replacing the media. Cultures were allowed to express for 24 to 96 h at 30°C or 37°C and 275 rpm. Strains JSIY017 and SPIY01 were grown in YPD rich growth media in 250 mL flasks, separated from the supernatant, and expressed in YPG rich expression media in 1.5 L flasks for 48 h at 175 rpm.
Activity Assay and Protein Concentration Determination
To detect extracellular β-glucosidase levels and determine kinetic activity parameters of the C75S variant, enzymatic colorimetric assay was carried out at 90°C using a thermostated spectrophotometer (Model DU600, Beckman, Fullerton, CA) as described previously 5. Volumes of 100 mM sodium acetate, pH 5.5 were heated to 90°C before adding p-nitrophenol-β-glucopyranoside (p-NPG) and protein. Baseline buffer spectra were subtracted. Vmax and Km were determined from Lineweaver-Burk plots by measurement of the hydrolysis of p-NPG at varying substrate concentrations at 90°C 15 of purified protein. Purified protein concentration was determined spectrophotometrically from the absorbance at 280 nm at pH 6.5 using a cell with a light path length of 1 cm and a molar extinction coefficient ∈280 of 128 280 M-1 cm-116.
Western Blot Detection of PDI and Intracellular and Secreted β-glucosidase
To prepare cell extracts, 1 OD-mL cells were separated by centrifugation at 2000 g for 5 min. After decanting the supernatant, the cell pellet was resuspended in 50 μL of lysis buffer (2% SDS, 90 mM HEPES, pH 7.5, 30 mM DTT, and 0.4 mg/ml Pefabloc, Sigma-Aldrich), and heated to 95°C for 10 min. The insoluble fraction was separated by centrifugation at 2000 g for 5 min. The soluble cell extracts were then added to 150 μL of resuspension buffer (3% SDS, 100 mM Tris base, 3 mM DTT, pH 11). Equal OD equivalent volumes of reduced cell extracts were separated by SDS-PAGE. To detect extracellular β-glucosidase, equal volumes of supernatant, after cells were removed by centrifugation, were separated by SDS-PAGE. Intracellular PDI was detected by a commercial monoclonal PDI antibody (Stratagene) (1:1000 dilution) with goat anti-mouse secondary antibody conjugated to horseradish peroxidase (Amersham Biosciences) (1:1000 dilution). Intracellular and extracellular β-glucosidase was detected by Western blot analysis using anti-c-myc polyclonal primary antibody (Covance) (1:1000 dilution) with goat anti-rabbit secondary antibody conjugated to horseradish peroxidase (Amersham Biosciences) (1:1000 dilution). Signals were visualized using ECL Plus detection reagents (Amersham Biosciences) and imaged with a FluorImager 595 (Molecular Dynamics). Images were quantitated using ImageQuant software Version 5.2 (Molecular Dynamics).
Results
Strain Construction and C75S β-glucosidase Activity Characterization
Previous studies showed that when P. furiosus β-glucosidase is overexpressed using the high copy transformant JSIY017, high levels of misfolded β-glucosidase accumulate in the ER, blocking the secretion pathway after approximately 12 h of expression 5. Additionally, while single additional copies of BiP and/or PDI showed modest improvement in reducing the secretion bottleneck, when multiple copies of BiP and PDI (with expression controlled by constitutive and inducible promoters) were tested, BiP decreased secretion while PDI increased yields approximately threefold 6. This result was unexpected since β-glucosidase contains no disulfide bonds in the native state. To determine whether PDI was recruited via the lone cysteine residue of β-glucosidase by forming a transient PDI-β-glucosidase complex, the single cysteine was mutated to a serine residue. After confirming only the selected residue was changed, a single copy of both pRS314-β and pRS314-βC75S were successfully introduced into both BJ5464 and hPDI (a strain with a high-level constitutive expression of PDI) 13.
Multiple copies of the C75S mutant gene tagged for secretion were also transformed into BJ5464 using the pITy-4-βC75S plasmid and grown and expressed in rich media as described in Materials and Methods. The supernatant was collected and C75S-β-glucosidase was successfully purified to homogeneity using the same four step method developed previously for wild-type β-glucosidase: removing cells from the supernatant, concentrating supernatant using 100,000 MWCO cross flow membranes (Sartorius, VivaScience), separating on DEAE-Sepharose ion-exchange column (GE-Amersham Biosciences), and resolving purified β-glucosidase with a gel filtration column (Superdex 200 10/30, GE-Amersham Biosciences)16.
Native-PAGE confirmed the same predominantly tetrameric quaternary structure as seen for the wild-type protein at room temperature (Figure 1b). The kinetic parameters Vmax and Km were determined by hydrolysis of p-NPG to ensure the variant maintained proper function (Table 1). These parameters were determined by linear extrapolation using the method of Kengen et al.15 to enable comparison to β-glucosidase derived from P. furiosus determined previously. The single mutation had little effect on the reaction rate as measured at 90°C, with a measured Vmax of 279 U/mg compared to 289 U/mg for wild type. Km values varied slightly from 0.54 mM for C75S compared to 0.34 mM for wild type. Because Km is based on a linear extrapolation from experimental data, it is not unusual for values to vary two-fold, and additionally the values are within the range calculated from the standard error of the fitted parameters; therefore the specificity and the active site of the protein was considered not to be disturbed substantially in the C75S variant. For comparison, wild-type β-glucosidase expressed and purified directly from P. furiosus has a Vmax of 700 U/mg and a Km of 0.15 mM 15. The kinetic parameters for β-glucosidase containing no epitope tags expressed in a recombinant E. coli system in our laboratory were similar: Vmax of 718 U/mg, Km of 0.33 mM (Smith, J.D. and Robinson, A.S., unpublished data). Therefore the difference in the maximum catalytic rate of β-glucosidase produced in these systems versus the yeast expression system is likely due to the extra linker and c-myc tag on the C-terminus added to the yeast expression systems to enable Western blot detection. These amino acid additions are believed to affect tetramer association but not the active site of the protein as they decrease Vmax but have little effect on Km.
Table 1.
Wild-type and C75S β-glucosidase secreted from Saccharomyces cerevisiae and purified (see Materials and Methods) have similar Michaelis-Menten kinetic parameters. One unit (U) is defined as the amount required to catalyze the formation of 1 mmole p-nitrophenol/min at 90°C. Shown also for comparison are kinetic parameters measured from β-glucosidase recombinantly expressed in E. coli, lacking the c-myc detection tag located on the C-terminus of each monomer a) Smith, J.D., and Robinson, A.S., unpublished data, and β-glucosidase purified directly from Pyrococcus furiosus b) 15.
| Vmax (U/mg protein) | Km (mM) | |
|---|---|---|
| wt β-glucosidase a | 289 | 0.34 |
| C75S β-glucosidase
(Range based on standard error of fitted parameters) |
279
(182-867) |
0.54
(0.4-1.4) |
β-glucosidase expressed in E. colia

|
718 | 0.33 |
β-glucosidase purified from P. furiosusb

|
700 | 0.15 |
C75S β-glucosidase Expression Characterization
To examine the effect of increasing PDI levels on wild-type and C75S β-glucosidase, two colonies from each transformant with a single gene copy, BJ wt, hPDI wt, BJ C75S, and hPDI C75S, were grown separately in 25 mL flasks as described in Materials and Methods. As expected, an increase in β-glucosidase secretion upon induction in cells with higher PDI levels was seen in samples extracted every 24 h for 96 h (Figure 2a). Because a single copy β-glucosidase plasmid selected over several generations was used in a well-characterized strain, there was not much colony-to-colony variation and experimental data is an average of the two colonies selected from each transformant. The difference between secretion levels described here and secretion described previously 6 is due to the use of different strains, as the strains used in this work have been well characterized for PDI concentration, while strains used previously were optimized for maximal β-glucosidase production. Trends are similar; PDI always improves β-glucosidase secretion at 30°C. Even by 24 h, the amount of secreted β-glucosidase is larger for the hPDI expressing strains than for the wild-type strains. This trend consistently continues with increased levels in hPDI strains up to the final time point measured at 96 h. Secretion improvement at high PDI levels was between 13 to 43% higher than wild-type (BJ) cells over the course of expression. PDI overexpression levels were steady after 48 h at approximately 12-15 fold higher than wild-type cells during expression of β-glucosidase, as determined by quantitative Western immunoassay (Table 2, Figure 2b). Intracellular β-glucosidase levels were also analyzed by quantitative Western immunoassay, but the difference seen between strains was slight, as the intracellular pool of misfolded β-glucosidase appears to be saturated (Figure 2b). This indicates that the differences observed in secretion were not due to large differences in expression. Surprisingly, little difference was observed between wild-type and C75S variant β-glucosidase secretion (Figure 2a).
Figure 2.

Time related overexpression of PDI at 30°C shows increased secretion of both wild-type and C75S β-glucosidase after 24 h until the final time measured at 96 h. Strains grown for 48 h in 25 mL SD-2xSCAA dextrose liquid growth media minus tryptophan supplements with 1 mg/mL BSA were separated from SD media by centrifugation and diluted to 1 OD-mL in 25 mL SG-2xSCAA galactose liquid expression media minus tryptophan supplements. At each time point, a supernatant fraction was separated by centrifugation and a constant volume was assayed for secreted β-glucosidase by activity. Additionally, 1 OD-mL equivalent was collected and intracellular β-glucosidase and intracellular PDI were detected by Western blotting with anti-c-myc polyclonal or anti-PDI monoclonal antibodies. a) Secreted wild-type and C75S β-glucosidase measured by activity assay of supernatant fractions, with filled circles representing BJ wt, open circles hPDI wt, filled square BJ C75S, and open squares hPDI C75S, normalized per cell OD. Data represents average of two independently selected transformants. b) Western blot detection of PDI and β-glucosidase in the intracellular fraction over 96 h time course. Lanes marked 1-4 correspond to a single transformant each of BJ wt, hPDI wt, BJ C75S, hPDI C75S, respectively at 24 h, 48 h, 72 h, and 96 h, loaded in equal OD equivalent volumes.
Table 2.
Comparison of relative PDI and intracellular β-glucosidase levels during β-glucosidase expression in different yeast strains after exponential growth phase (48 h) in batch culture normalized to wild-type β-glucosidase in BJ5464. 1 OD-mL yeast cells expressing β-glucosidase in 25 mL batch culture were collected and lysed as described in Materials and Methods. Cell lysates were subjected to SDS-PAGE and the intracellular PDI was detected by Western blotting. The PDI levels relative to the parent strain BJ5464+pRS314-β were determined using ImageQuant software (Molecular Dynamics). The range of PDI levels is based on five sets of Western blot data.
| PDI level during expression in BJ strain (relative to wt) | PDI level during expression in hPDI strain (relative to wt) | Intracellular β-glucosidase in BJ strain (relative to wt) | Intracellular β-glucosidase in hPDI strain (relative to wt) | |
|---|---|---|---|---|
| wt β-glucosidase | 1 | 12-15 | 1 | 0.8-1.2 |
| C75S β-glucosidase | 1-1.3 | 12-15 | 0.8-1.2 | 0.8-1.2 |
PDI Assists Secretion of Both Wild-type and C75S
As the increases in secretion seen with increased PDI were consistent but relatively modest, further analysis of the same colonies was carried out for the 48 h time point. At 48 h, cell growth was healthy, while Western blots detecting extracellular β-glucosidase taken at 72 and 96 h show degradation of secreted protein by detection of smaller fragments (not shown), indicating some cell lysis.
To show the increase in β-glucosidase secretion and limit error in interpretations based on experimental variation, particularly from variations across different Western blots, at least six independent expressions of the four transformants were measured. Western blot analysis showed that extracellular β-glucosidase levels were higher when PDI levels were increased for both the wild-type and C75S variant (Figure 3), signifying that increased PDI levels have a positive effect on β-glucosidase secretion at 30°C, even for the redox-inactive variant. Using a 98% confidence interval, a paired t-test of this data confirms the hypothesis that the wild-type strain expresses less β-glucosidase than the PDI overexpression strain, with a p-value of .032 for the wild-type β-glucosidase, and .007 for the C75S variant, showing the likelihood this hypothesis is incorrect is less than 3.2% or 0.7%, respectively. Although less pronounced, the secretion level as determined by activity in the supernatant from the same expression runs increased for both the wild-type and C75S variant approximately two-fold with increased PDI levels, with p-values (98% confidence) of .011 and .000, respectively (Figure 3). The figures shown for 30°C expression are from nine independent experiments of each transformant. Results are normalized per cell OD, but the average cell OD for each transformant was within the range of 15 ± 0.5. While the C75S appeared to have a slightly higher secretion as detected by activity assay at 30°C, it was not as pronounced as the effect due to PDI. Additionally, no effect was seen when Western blot data was compared. Thus we conclude that no major effect of the mutation itself on secretion is observed.
Figure 3.
PDI increases the secretion of both wild-type and C75S β-glucosidase at 30°C as detected by Western blot analysis and activity assay. Single colonies of BJ wt, hPDI wt, BJ C75S, and hPDI C75S were grown for 48 h in 5 mL SD-2xSCAA dextrose liquid growth media minus tryptophan supplements at 30°C. SD media was removed by centrifugation, and the cell pellet was resuspended to 1 OD-mL in 5 mL SG-2xSCAA galactose liquid expression media minus tryptophan supplements. After expression for 48 h at 30°C, extracellular β-glucosidase levels were detected in a constant volume of supernatant separated from cells by centrifugation by Western blot analysis (filled bars) using an anti-c-myc polyclonal antibody for each expression and normalized per cell OD. 20 μL of the supernatant was separated by centrifugation and assayed for active extracellular β-glucosidase (open bars), normalized per cell OD. The average of nine independent expressions is plotted and the error bar represents the standard error of the measurement.
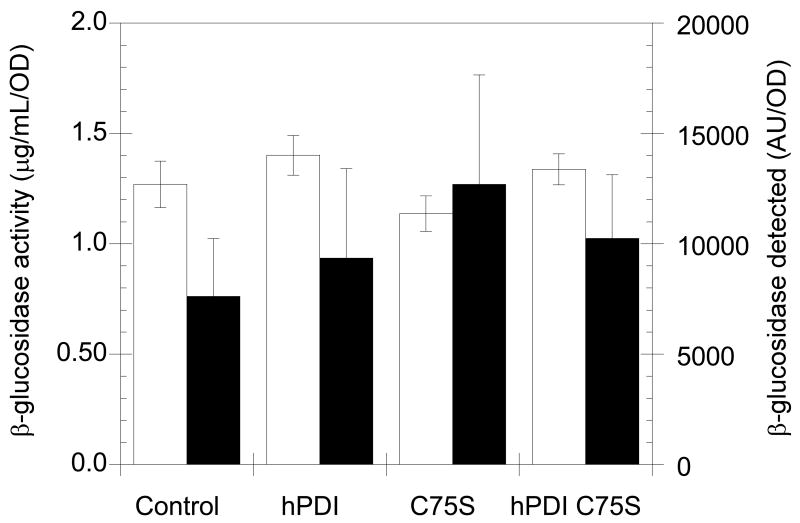
At 37°C, a larger quantity of protein is secreted, as expected for both the wild-type and C75S variants, since the cells are healthier and the secretion bottleneck is alleviated 7. PDI no longer has an effect on secretion as detected by Western blot analysis; both wild-type and C75S secrete equally well (Figure 4). While secreted β-glucosidase is slightly higher as measured by activity assay (from around 1.25 mg/mL/OD to 1.4 mg/mL/OD) in the high PDI strains (Figure 4), this difference is not nearly as distinct as the effect at 30°C. The C75S mutation shows no statistical difference in secretion compared to wt with increased PDI levels. The figures shown are averages of six independent experiments of each transformant.
Figure 4.
Overexpression of PDI does not increase the secretion of either wild-type or C75S β-glucosidase at 37°C where the ER secretion bottleneck is removed as detected by activity assay. Single colonies of BJ wt, hPDI wt, BJ C75S, and hPDI C75S were grown for 48 h in 5 mL SD-2xSCAA dextrose liquid growth media minus tryptophan supplements at 30°C. SD media was removed by centrifugation, and the cell pellet was resuspended to 1 OD-mL in 5 mL SG-2xSCAA galactose liquid expression media minus tryptophan supplements. After expression for 48 h at 37°C, extracellular β-glucosidase levels were detected in a constant volume of supernatant separated from cells by centrifugation by Western blot analysis (filled bars) using an anti-c-myc polyclonal antibody for each expression and normalized per cell OD. 20 μL of the supernatant was separated by centrifugation and assayed for active extracellular β-glucosidase (open bars), normalized per cell OD. The average of six independent expressions is plotted, and the error bar represents the standard error.
As we are optimizing this system to identify possible methods to produce large quantities of hyperthermophilic proteins for industrial uses, we were interested in whether PDI would still have a positive effect after the scale-up of expression volume. We increased the total volume five-fold to 25 mL and repeated the analysis of expression at 30°C. Extracellular β-glucosidase levels were still comparably higher when PDI levels were increased for both the wild-type and C75S variant by detection with both Western blot and activity assay (data not shown).
Discussion
Altering the concentrations of two ER-resident proteins, PDI and BiP, has been shown to minimize overloading of the yeast host's machinery 3, 17, 18 during expression and secretion of a foreign protein. In the expression system for P. furiosus β-glucosidase developed previously in our lab, addition of multiple copies of PDI and BiP showed that increased BiP levels overall decreased secretion, but increased PDI levels produced up to a threefold higher yield. To evaluate the role of PDI and its interaction with β-glucosidase during the folding process, a protein variant was constructed with the cysteine residue replaced by a serine, which lacks the thiol functionality.
Overexpression of PDI improves secretion for both wild-type and C75S β-glucosidase. While PDI levels were slightly greater than wild-type in the cell line expressing C75S β-glucosidase (Table 1), this could be due to experimental detection errors or possibly an improved folding rate. A greater difference in β-glucosidase production in strains with low and high levels of PDI is observed with Western detection compared to the activity assay. One possibility for this difference is that PDI is simply helping to shuttle the β-glucosidase outside the cell faster, and due to a shorter residence time in the ER, perhaps some additional slightly misfolded inactive protein is detected by Western blot. Both wild-type and C75S β-glucosidase bands on the SDS-PAGE gels are more smeared in the high PDI strains, suggesting a less uniform population.
Although the role of PDI as a chaperone is poorly understood, it has been clearly shown to bind to peptides and misfolded proteins 19 and act as a chaperone both in vivo and in vitro9, 10. While chaperone activity has been shown to aid in the refolding of a protein with no disulfide bonds 20, evidence of chaperone activity in a sulfhydryl-free protein has not yet been reported. In this system, even at high levels of PDI, only a beneficial role was seen 6, unlike its reported anti-chaperone activity 21, 22. PDI could also assist as a chaperone in oligomeric assembly, independent of proper monomer unit folding. PDI has previously been shown to function as a foldase in the refolding of the dimeric alkaline protease inhibitor (API) from Streptomyces23. In API, PDI was shown to suppress aggregation and maintain monomers in a folding-competent state. Even though the bottleneck in β-glucosidase is believed to be caused by aggregation of misfolded monomers rather than incorrect assembly, the role in forming proper tetrameric structure of β-glucosidase cannot be eliminated.
Another possibility is that PDI could be an interacting with the trafficking or degradation machinery of the cell either directly or indirectly as part of quality control for selecting and removing misfolded protein rather than directly assisting in folding. PDI has been previously shown to be involved in ER-associated degradation (ERAD). When a mutation of the cysteine-free secretory protein ppαf, Δppαf, was expressed in yeast cells with a mutant PDI lacking the putative binding region, properly folded protein was only marginally affected, but export for degradation was deficient. PDI was found to have a role in recognizing misfolded proteins and targeting them across the ER membrane to the cytosol 24. In β-glucosidase expression, the increase in secretion at high temperature is due to effects on β-glucosidase itself instead of temperature effects on the cell 7. By encouraging proper folding during expression at higher temperature, the bottleneck is relieved, and perhaps PDI is no longer needed for ERAD so no positive effects are seen at increased PDI levels.
Even though PDI increases secretion of a redox-inactive protein, further experimental evidence is needed to determine whether PDI and β-glucosidase are interacting directly. While co-immunoprecipitation experiments have not shown any indication that β-glucosidase and PDI are directly associated with these strains and this experimental platform, further study should lead to an improved understanding of the role of PDI and its chaperone activity in folding and secretion.
Conclusions
We have shown that a protein does not have to be redox active for PDI to assist in secretion from the ER. We also have shown that once the folding bottleneck is relieved, which for this protein occurs when the expression temperature is increased to 37°C, PDI no longer has a beneficial effect. While we cannot yet say whether increases in secretion at 30°C are due to direct interaction between PDI and β-glucosidase, or due to indirect interaction with the folding or degradation machinery of the cell, we have shown that optimizing PDI expression levels is a beneficial approach for increasing recombinant overexpression of even sulfhydryl-free proteins.
Acknowledgments
We would like to thank Dr. Clifford Robinson, Dr. Jason Smith, Ping Xu, Dr. David Raden, Steven Bane, and Michelle O'Malley for experimental assistance and helpful discussions. We would also like to thank S.J.J. Brouns, T. Kaper, W.M. de Vos and J. van der Oost for the P. furiosus β-glucosidase structural coordinates that aided in this study. Funding for this work was provided in part by NIH T32 GM 08550-09 (SLP), NSF BES99-84312, and UDRF.
This study was supported by NIH T32 GM 08550-09 (SLP), NSF BES99-84312, and the University of Delaware Research Foundation.
References
- 1.Mozhaev VV. Mechanism-based strategies for protein thermostabilization. Trends in Biotechnology. 1993;11:88–95. doi: 10.1016/0167-7799(93)90057-G. [DOI] [PubMed] [Google Scholar]
- 2.Adams MW, Perler FB, Kelly RM. Extremozymes: expanding the limits of biocatalysis. Biotechnology (N Y) 1995;13(7):662–8. doi: 10.1038/nbt0795-662. [DOI] [PubMed] [Google Scholar]
- 3.Robinson AS, Wittrup KD. Constitutive overexpression of secreted heterologous proteins decreases extractable BiP and protein disulfide isomerase levels in Saccharomyces cerevisiae. Biotechnol Prog. 1995;11(2):171–7. doi: 10.1021/bp00032a009. [DOI] [PubMed] [Google Scholar]
- 4.Fiala G, Stetter KO. Pyrococcus furiosus sp. nov. represents a novel genus of marine heterotrophic archaebacteria growing optimally at 100°C. Archives of Microbiology. 1986;145:56–61. [Google Scholar]
- 5.Smith JD, Robinson AS. Overexpression of an archaeal protein in yeast: secretion bottleneck at the ER. Biotechnol Bioeng. 2002;79(7):713–23. doi: 10.1002/bit.10367. [DOI] [PubMed] [Google Scholar]
- 6.Smith JD, Tang BC, Robinson AS. Protein disulfide isomerase, but not binding protein, overexpression enhances secretion of a non-disulfide-bonded protein in yeast. Biotechnol Bioeng. 2004;85(3):340–50. doi: 10.1002/bit.10853. [DOI] [PubMed] [Google Scholar]
- 7.Smith JD, Richardson NE, Robinson AS. Elevated expression temperature in a mesophilic host results in increased secretion of a hyperthermophilic enzyme and decreased cell stress. Biochim Biophys Acta. 2005;1752(1):18–25. doi: 10.1016/j.bbapap.2005.07.016. [DOI] [PubMed] [Google Scholar]
- 8.Laboissiere MC, Sturley SL, Raines RT. The essential function of protein-disulfide isomerase is to unscramble non-native disulfide bonds. J Biol Chem. 1995;270(47):28006–9. doi: 10.1074/jbc.270.47.28006. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert HF. Protein disulfide isomerase. Methods Enzymol. 1998;290:26–50. doi: 10.1016/s0076-6879(98)90005-2. [DOI] [PubMed] [Google Scholar]
- 10.Wang CC. Protein disulfide isomerase as an enzyme and a chaperone in protein folding. Methods Enzymol. 2002;348:66–75. doi: 10.1016/s0076-6879(02)48627-2. [DOI] [PubMed] [Google Scholar]
- 11.Xiao R, Wilkinson B, Solovyov A, Winther JR, Holmgren A, Lundstrom-Ljung J, Gilbert HF. The contributions of protein disulfide isomerase and its homologues to oxidative protein folding in the yeast endoplasmic reticulum. J Biol Chem. 2004;279(48):49780–6. doi: 10.1074/jbc.M409210200. [DOI] [PubMed] [Google Scholar]
- 12.Parekh RN, Shaw MR, Wittrup KD. An integrating vector for tunable, high copy, stable integration into the dispersed Ty delta sites of Saccharomyces cerevisiae. Biotechnol Prog. 1996;12(1):16–21. doi: 10.1021/bp9500627. [DOI] [PubMed] [Google Scholar]
- 13.Xu P, Raden D, Doyle FJ, 3rd, Robinson AS. Analysis of unfolded protein response during single-chain antibody expression in Saccaromyces cerevisiae reveals different roles for BiP and PDI in folding. Metab Eng. 2005;7(4):269–79. doi: 10.1016/j.ymben.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 14.Wittrup KD, Benig V. Optimization of amino-acid supplements for heterologous protein secretion in Saccharomyces cerevisiae. Biotechnology Techniques. 1994;8(3):161–166. [Google Scholar]
- 15.Kengen SW, Luesink EJ, Stams AJ, Zehnder AJ. Purification and characterization of an extremely thermostable beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur J Biochem. 1993;213(1):305–12. doi: 10.1111/j.1432-1033.1993.tb17763.x. [DOI] [PubMed] [Google Scholar]
- 16.Powers SL, Robinson CR, Robinson AS. Denaturation of an extremely stable hyperthermophilic protein occurs via a dimeric intermediate. Extremophiles. 2007;11:179–189. doi: 10.1007/s00792-006-0030-5. [DOI] [PubMed] [Google Scholar]
- 17.Robinson AS, Bockhaus JA, Voegler AC, Wittrup KD. Reduction of BiP levels decreases heterologous protein secretion in Saccharomyces cerevisiae. J Biol Chem. 1996;271(17):10017–22. doi: 10.1074/jbc.271.17.10017. [DOI] [PubMed] [Google Scholar]
- 18.Robinson AS, Hines V, Wittrup KD. Protein disulfide isomerase overexpression increases secretion of foreign proteins in Saccharomyces cerevisiae. Biotechnology (N Y) 1994;12(4):381–4. doi: 10.1038/nbt0494-381. [DOI] [PubMed] [Google Scholar]
- 19.Klappa P, Ruddock LW, Darby NJ, Freedman RB. The b' domain provides the principal peptide-binding site of protein disulfide isomerase but all domains contribute to binding of misfolded proteins. Embo J. 1998;17(4):927–35. doi: 10.1093/emboj/17.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai H, Wang CC, Tsou CL. Chaperone-like activity of protein disulfide isomerase in the refolding of a protein with no disulfide bonds. J Biol Chem. 1994;269(40):24550–2. [PubMed] [Google Scholar]
- 21.Primm TP, Walker KW, Gilbert HF. Facilitated protein aggregation. Effects of calcium on the chaperone and anti-chaperone activity of protein disulfide-isomerase. J Biol Chem. 1996;271(52):33664–9. doi: 10.1074/jbc.271.52.33664. [DOI] [PubMed] [Google Scholar]
- 22.Quan H, Fan G, Wang CC. Independence of the chaperone activity of protein disulfide isomerase from its thioredoxin-like active site. J Biol Chem. 1995;270(29):17078–80. doi: 10.1074/jbc.270.29.17078. [DOI] [PubMed] [Google Scholar]
- 23.Pandhare J, Deshpande V. Both chaperone and isomerase functions of protein disulfide isomerase are essential for acceleration of the oxidative refolding and reactivation of dimeric alkaline protease inhibitor. Protein Sci. 2004;13(9):2493–501. doi: 10.1110/ps.03552004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillece P, Luz JM, Lennarz WJ, de La Cruz FJ, Romisch K. Export of a cysteine-free misfolded secretory protein from the endoplasmic reticulum for degradation requires interaction with protein disulfide isomerase. J Cell Biol. 1999;147(7):1443–56. doi: 10.1083/jcb.147.7.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]