Abstract
The group 1 allergens are a major cause of cedar pollen hypersensitivity in several geographic areas. Allergens from several taxa have been shown to cross-react. The goal of these studies was to compare the structural features of the shared and unique epitopes of the group 1 allergen from mountain cedar (Jun a 1) and Japanese cedar (Cry j 1). An array of overlapping peptides from the sequence of Jun a 1 and a panel of monoclonal anti-Cry j 1 antibodies were used to identify the IgE epitopes recognized by cedar-sensitive patients from Texas and Japan. IgE from Japanese patients reacted with peptides representing one of the two linear epitopes within the highly conserved β-helical core structure and both epitopes within less ordered loops and turns near the N- and C-termini of Jun a 1. A three-dimensional (3D) model of the Cry j 1, based on the crystal structure of Jun a 1, indicated a similar surface exposure for the four described epitopes of Jun a 1 and the homologous regions of Cry j 1. The monoclonal antibodies identified another shared epitope, which is most likely conformational and a unique Cry j 1 epitope that may be the previously recognized glycopeptide IgE epitope. Defining the structural basis for shared and unique epitopes will help to identify critical features of IgE epitopes that can be used to develop mimotopes or identify allergen homologues for vaccine development.
Keywords: Allergy, Allergen structure, Cedar pollen hypersensitivity, Cry j 1, Cryptomeria japonica, IgE epitope, Jun a 1, Juniperus ashei, MPACK
1. Introduction
Cedar pollen hypersensitivity is a major cause of seasonal airway symptoms in several regions of the Northern Hemisphere. Group 1 allergens, which are structurally similar to bacterial pectate and pectin lyases (Czerwinski et al., 2005), have been isolated from pollens of mountain cedar (Jun a 1, Juniperus ashei, Cupressaceae; Midoro-Horiuti et al., 1999), eastern red cedar (Jun v 1, Juniperus virginiana, Cupressaceae; Midoro-Horiuti et al., 2001), Italian cypress (Cup s 1, Cupressus sempervirens, Cupressaceae; Arilla et al., 2004), Japanese cypress (Cha o 1, Chamaecyparis obtusa, Cupressaceae; Suzuki et al., 1996) and Japanese cedar (Cry j 1, Cryptomeria japonica, Taxodiaceae; Yasueda et al., 1983). A substantial proportion of the IgE anti-cedar pollen antibodies from patients with mountain cedar allergy react with Cry j 1 (Midoro-Horiuti et al., 1999; Taniai et al., 1993). Thus, the high degree of sequence identity between group 1 allergens could account for the cross-reactivity between these pollens, as demonstrated by skin testing with crude extracts (Schwietz et al., 2000). In fact, we have previously reported cross-reactivity between serum IgE antibodies to Jun a 1 and Cry j 1 (Midoro-Horiuti et al., 1999). However, the molecular basis for this cross-reactivity has yet to be elucidated.
We recently identified three major and one minor epitope of Jun a 1, using overlapping, synthetic peptides and sera from patients allergic to mountain cedar pollen (Midoro-Horiuti et al., 2003). Two of these epitopes are near the highly conserved pectate lyase catalytic site, making it likely that these epitopes are shared among many or all cedars (Midoro-Horiuti et al., 2003). The high-resolution crystal structure of Jun a 1 (Czerwinski et al., 2005), the first for any cedar allergen, identified a β-helical core structure that is highly conserved between the microbial pectate and pectin lyases and this plant protein. However, there are substantial structural differences in the loops and turns that protrude from their cores. While the degree of amino acid sequence identity is very high among the plant pectate lyase-like proteins, the structural and functional similarities between these group 1 allergen remains to be elucidated.
The goal of the present study was to use our understanding of the structure of IgE epitopes of Jun a 1 to identify similar regions in Cry j 1 and determine the extent to which these structures represent shared and unique epitopes in the IgE responses to these homologous group 1 allergens. Thus, synthetic peptide arrays, designed from the amino acid sequence of Jun a 1, were tested for their binding of IgE in sera from patients sensitive to Japanese cedar pollen and of mouse monoclonal anti-Cry j 1 antibodies. Using our previously developed modeling tools (Soman et al., 2000; Schein et al., 2001; Ivanciuc et al., 2004, 2002, 2003; Murtazina et al., 2002), we prepared a 3D-model of the structure of Cry j 1, based on the crystal structure of Jun a 1 and mapped the linear epitopes on the model structure. This approach allowed us to identify both shared and unique linear and probable conformational epitopes of these two group 1 cedar allergens.
2. Material and methods
2.1. Allergen preparation
Jun a 1 was purified from mountain cedar pollen (Bayer) and Cry j 1 from Japanese cedar (collected in Tamano City, Japan) using Con-A Sepharose 4B (Pharmacia) chromatography, as described previously (Midoro-Horiuti et al., 1999), and dialyzed against 0.05 M Tris–HCl buffer, pH 7.8, prior to further analysis.
2.2. Human sera and mouse mAb
Sera from 11 mountain cedar allergic patients were obtained from volunteers from the Austin, Texas area. Two sets of sera from Japanese cedar allergic patients were tested. One set was collected from Fukuyama as described previously (age 7–10 years, 8.5 ± 1.3), male/female = 1/3, Midoro-Horiuti et al., 1999) and a new set of 17 sera (age 3–52 years, 19.2 ± 14.1), male/female = 6/11 were collected from volunteers in Nagoya, Japan. The diagnosis of seasonal allergic rhinitis due to cedar sensitivity was established in all subjects by clinical history and CAP-RAST (Pharmacia) or scratch skin testing results. Sera from the Nagoya panel were selected for analysis based on their reactivity to Cry j 1 in allergen-specific CAP-RAST.
Six monoclonal antibodies (mAbs) against native Cry j 1 (S14, S36, S84, S91, S95 and S131) were described previously (Sakaguchi et al., 1997). These antibodies represent four of the five antibody groups defined by Sakaguchi et al, including the three mAbs groups that competed with the IgE in the sera of Japanese cedar-sensitive patient for binding to Cry j 1.
2.3. Binding of IgE to Japanese cedar and mountain cedar pollen extracts, and inhibition with Cry j 1 and Jun a 1
ImmunoCAP assays (Pharmacia) were performed to quantify the binding of serum IgE from 17 Japanese cedar hypersensitivity patients to Japanese cedar and mountain cedar pollen extracts. Those sera with >0.70 U/ml of IgE to either pollen were used for inhibition assay. ImmunoCAP inhibition assays were performed to estimate the contribution of Jun a 1 and Cry j 1-reactive IgE to the response to pollen extracts. Patients’ sera were preincubated with a final concentration of 0.25 mg/ml of purified Cry j 1 or Jun a 1 or buffer control. After overnight preincubation at 4 °C, IgE binding to Japanese cedar extracts was quantified using the Immuno-CAP assay. The percent inhibition of IgE binding by Cry j 1 or Jun a 1 was calculated by the formula: % inhibition = [(serum alone − serum pre-incubated with Cry j 1 or Jun a 1)/serum alone] × 100. The results were expressed as the range and (mean ± standard deviation).
2.4. Dot blot immunoassay for antibody binding to Jun a 1 and its overlapping, synthetic peptides
The reactivity of anti-Cry j 1 mAbs with purified, native Jun a 1 was analyzed using dot blot analysis. Proteins (1 μg) were dotted on a cellulose membrane and dried. Membranes were incubated with blocking buffer (Sigma Genosys) containing 10% sucrose. Sections of the membrane were incubated with 2 μg/ml of each mAb. The washed membranes were then incubated with biotinylated anti-mouse IgG (Zymed). After incubation with HRP-streptavidin (Vector), membranes were placed in Enhanced Chemiluminescence (ECL, Pharmacia) to visualize the signal.
Individual 13 amino acid peptides, based on the Jun a 1 amino acid sequence (7), with eight amino acid overlaps were synthesized on a derivatized cellulose membrane, using N-(9-fluorenyl)methoxycarbonyl(Fmoc) amino acid active esters by Sigma Genosys. The binding of IgE from patients’ sera was analyzed as described previously (Midoro-Horiuti et al., 2003). Briefly, we used two serum pools, each with four different sera from Japanese children who demonstrated reactivity with Jun a 1 in ELISA, followed by biotinylated goat anti-human IgE (Vector) as the second antibody. The membranes were developed with HRP-streptavidin and ECL (Pharmacia). The intensity of the IgE binding to individual peptides on photographic images was scored visually on a scale of 0–3.
To identify the minimal linear structures required for IgE binding to each epitope, detailed epitope mapping was performed using another set of synthetic, overlapping peptides. These 13 or 8 amino acids long peptides were designed based on the sequences of the original 13-mer peptides that bound IgE. Each of these peptides overlapped each other by either 11 or 6 residues, resulting in a set of peptides that differed by only two amino acids.
To identify the epitopes recognized by anti-Cry j 1 mAbs KW-S84, 91, 95 and 131, the membranes were incubated overnight with 2 μg/ml of each mAb in blocking buffer. Bound IgG was detected with HRP conjugated anti-mouse IgG (Zymed) and ECL.
2.5. Inhibition of IgE-binding to Jun a 1 by mAbs
Jun a 1-coated plates were washed and incubated with 20 μg/ml of mAbs, KW-S84, 91, 95 and 131, at room temperature for 2 h. Next, individual Texas sera were added to the same wells and incubated at room temperature for 1 h. The binding of human IgE was quantified as described previously (Midoro-Horiuti et al., 1999). Results were expressed as the range of the percent inhibition of IgE binding, compared to wells which did not contain any mAb, and computed by the formula: % inhibition = [(serum alone − serum after mAb)/(serum alone)] × 100.
2.6. Homology modeling of Cry j 1
The amino acid sequence of Cry j 1 is 78.9% identical to that of Jun a 1. A model of Cry j 1 was prepared using coordinates of the Jun a 1 crystal structure as the template (Czerwinski et al., 2005) and the MPACK modeling suite (Ivanciuc et al., 2004; Midoro-Horiuti et al., 2003; Soman et al., 2001). The structure shown has the lowest target function of the 20 calculated by the self-correcting distance geometry program DIAMOD. For the modeling, disulfides were specified as constraints between residues C7–C24, C107–C126 and C285–C291 based on their identity between the template and the target. The backbone root-mean-square deviation (RMSD) of the model to the template is 0.38 Å. The GETAREA program of our FANTOM program was used to determine residue surface exposure (Fraczkiewicz and Braun, 1998). Graphical representation of the model and determination of nearest neighbor atoms was done with the MOLMOL program (Koradi et al., 1996).
3. Results
3.1. Direct and cross-reacting IgE antibodies in Japanese
The sera from patients from Nagoya, who were allergic to Japanese cedar and were known to be reactive to Cry j 1 were tested for IgE binding to Japanese cedar and mountain cedar extracts. As shown in Fig. 1, 13 out of 17 sera from Japanese cedar sensitive patients (76.5%) contained detectable amounts of IgE that reacted with mountain cedar extracts (Fig. 1). Regression analysis indicated that the extent of IgE binding to the Japanese cedar and mountain cedar extract were significantly correlated (r2 = 0.35 and p = 0.04).
Fig. 1.
IgE to Japanese cedar and mountain cedar. The concentration of IgE antibodies in sera from Japanese cedar sensitive patients that react with Japanese cedar and mountain cedar pollen were quantified by ImmunoCAP; r2 = 0.35, p = 0.04.
3.2. Purified group 1 allergens inhibit IgE binding to Japanese cedar pollen extracts
To quantify the extent of cross-reactivity between Jun a 1 and Cry j 1, ImmunoCAP inhibition assays were performed with purified, native allergens. Preincubation of sera with purified Cry j 1 inhibited 10.3–93.8% (54.1 ± 20.8)% of the binding of IgE from Japanese patients’ sera to Japanese cedar caps, while preincubation with purified Jun a 1 inhibited 0.5–42.3% (17.5 ± 12.5)% of the binding. The distribution of these values is shown in Fig. 2. Both Cry j 1 and Jun a 1 significantly inhibited IgE binding to Japanese cedar extracts (p < 0.0001, compared to buffer control). However, the degree of inhibition of individual sera by the two allergens was not correlated (r2 = 0.09, p = 0.27).
Fig. 2.

ImmunoCAP inhibition. Inhibition by purified Cry j 1 and Jun a 1 of the binding of IgE from Japanese patients to Japanese cedar extracts. Mean inhibition ± S.D. for Cry j 1 and Jun a 1 are shown with bars. The extent of inhibition by Cry j 1 and Jun a 1 was significantly greater that that by buffer control (p < 0.0001).
3.3. Binding of human and mouse antibodies to synthetic overlapping peptides define three cross-reactive IgE epitopes
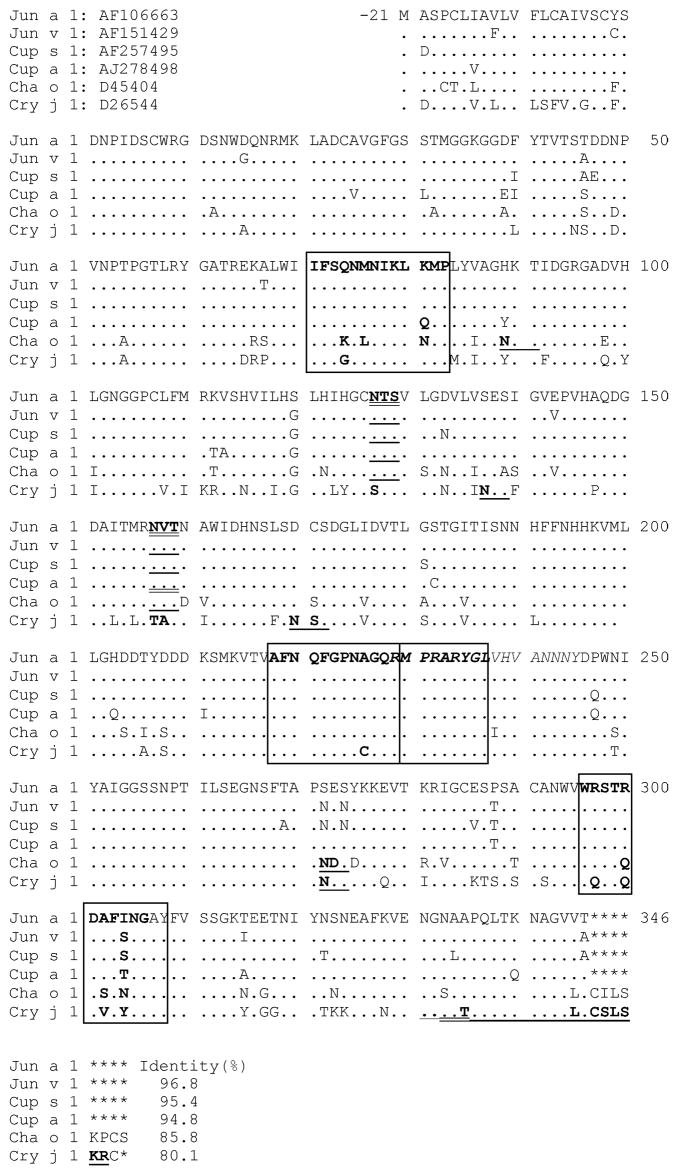
The sera from Fukuyama were tested for direct binding to Jun a 1 by dot blot analysis. All of the sera were positive in this assay. Two sets of four sera with the strongest reactivity with intact Jun a 1 were pooled and tested for binding to synthetic, Jun a 1 peptides. The pooled sera reacted to peptides Ile71-Pro83, Lys211-Gly223, Thr216-Gln228, Gln221-Ala233, Ala226-Val238 and Trp296-Tyr308, as shown in Fig. 3. The reactivity of the pooled sera from mountain cedar-sensitive patients is shown for comparison. These findings suggest that the display of these regions are similar in Jun a 1 and Cry j 1 and that the IgE in some Japanese cedar-sensitive patients react with Jun a 1 epitopes 1, 2 and 4 (but not 3).
Fig. 3.
IgE binding to overlapping peptides. (A) Epitope mapping was performed by testing the binding of serum IgE from individual Texas patients (left) and pooled sera from four Japanese patients (right) to overlapping synthetic peptides based on Jun a 1 sequence. (B) The sequences of the synthetic peptides are shown along with the antibody binding activity, scored 0–3+. The epitope regions are defined with boxes.
Antibodies representing three of the six groups of anti-Cry j 1 mAbs (S84, S91/S95 and S131) cross-reacted with intact Jun a 1. Two of these groups (S84 and S91/95) reacted with synthetic, overlapping peptides of Jun a 1. MAbs (S84) bound to a peptide that included the three N-terminal amino acids Ile71Phe72Ser73 of Jun a 1 peptides representing IgE epitope 1: Ile71-Pro83. The binding pattern of the other mAbs (S91 and S95) paralleled that of IgE reactivity with epitope 2 peptides: Ala218-Arg229 (Fig. 3A). These mAbs did not react with the epitope 3 peptide (Met230-Leu237). We previously divided the region Ala218-Leu237 into two distinct epitope areas, based on IgE reactivity (Midoro-Horiuti et al., 2003). The pattern of reactivity of these Cry j 1-specific mAb with the Jun a 1 peptides provides additional evidence that the region between residues Ala218-Arg229 contains a strong B-cell epitope that is distinct from that produced by the next eight residues (Met230-Leu237).
3.4. Definition of minimal IgE epitopes
To define the minimum span of amino acids required to form IgE epitopes recognized by Japanese cedar-sensitive patients, we used the same serum pool from Fukuoka to probe a second set of overlapping peptides that represented the sequences around epitopic regions 1–4, but differed from their contiguous peptides by only two residues. The results are shown in Fig. 4. For comparison, the results for a pool of sera from mountain cedar-sensitive patients are also shown. This particular pool reacted less intensely with the epitope 1 peptide (Ile71-Pro83) than the pool used previously (Midoro et al., 2003). Neither of the serum pools showed any reaction with the 13-mer peptides with 11 amino acids overlap to this area. Thus, the minimal IgE epitope 1 that could be defined was the 13-mer peptide Ile71-Pro83. Of the 10 8-mers representing the epitope 2 and3 region, two peptides, Phe222-Arg229 and Met230-Leu237 bound IgE from Texas sera most strongly (Midoro-Horiuti et al., 2003). There was also weaker reactivity with the peptides between these (Fig. 4). The IgE from Japanese cedar-allergic patients’ sera also reacted strongly with the Phe222-Arg229 peptide, but did not react with the Met230-Leu237peptide. This difference in reactivity of the two pools of sera clearly delineated IgE epitopes 2 and 3. IgE from both serum pools reacted with a single 13-mer that represents epitope 4 (Fig. 3). However, on detailed analysis, using 10-mer peptides, the sera from mountain cedar-sensitive patients recognized three overlapping peptides, while the sera from Japanese cedar patient only reacted only Trp296-Tyr308 (Fig. 4).
Fig. 4.
IgE antibody reactivity with overlapping peptides representing sequences around the four Jun a 1 epitopes defined in Fig. 3. (A) Probed with sera from mountain cedar (left) and Japanese cedar sensitive patients (right). (B) Sequences of the peptides to which IgE reacted are shown and the minimal epitopes are indicated by boxes.
3.5. Comparison of amino acid sequences of group 1 allergens from cedar pollen
The available amino acid sequences for the group 1 cedar and cypress allergens were compared with that of Jun a 1 (Fig. 5). There was an overall high degree in sequence identity (mean = 90.6%). Within the Jun a 1 epitope 1–4 regions, the mean percent identity was 92.3, 98.3, 100 and 81.8, respectively. Interestingly, even though the sequence of the epitope 3 region was identical for Jun a 1 and Cry j 1, only the sera from mountain cedar-sensitive patients reacted with the peptides representing that epitope.
Fig. 5.
Comparison of the sequences of the IgE epitope regions of Jun a 1 and those of other group 1 allergens. Four IgE epitopes are shown with boxes. Proven N-glycosylation sites are shown with double underlines and potential N-glycosylation sites are shown with single underlines. The PL active site is italicized. Jun v 1 is from eastern red cedar, Cup a 1 from Arizona cypress, Cup s 1 from Italian cypress, Cha o 1 from Japanese cypress and Cry j 1 from Japanese cedar. Previously reported IgE epitope Asn331-Arg352 was marked.
3.6. Inhibition of human IgE binding to Jun a 1 by mAbs to Cry j 1
MAbs KW-S84, S91, S95 and S131 were tested for their ability to inhibit the binding to Jun a 1 of IgE from the 11 mountain cedar-sensitive patients. Antibodies KW-S84, S91, S95 and S131 inhibited the binding of 0–16 (−1.6 ± 3.7), 0–15 (3.6 ± 11.6), 0–25 (15.6 ± 7.8) and 6–34 (18.6 ± 10.7)% of the Jun a 1-specific IgE antibodies, respectively. These findings and their potential relevance are summarized in Table 1.
Table 1.
Summary of epitopes of monoclonal antibodies and human IgE
| Cry j 1 epitope (Sakaguchi et al.) | Inhibition with mAb | Cross-reactivity with Jun a 1 | Inhibition of IgE binding (%) | Reactivity to Jun a 1 epitope | Interpretation |
|---|---|---|---|---|---|
| 1 (S14) | 21/21 | − | NT | - | Cry j 1 unique epitope (glycopeptide?) |
| 2 (NT) | 0/21 | NT | NT | - | Mouse epitope |
| 3 (S131) | 11/21 | + | 6–34 | - | Strong epitope in mice and human conformational? |
| 4 (S84) | 0/21 | + | 0–16 | Near epitope 1 | Mouse epitope |
| 5 (S95) | 10/21 | + | 0–25 | Epitope 2 | Strong epitope in mice and human linear |
3.7. Predicted structure of the IgE epitope on Cry j 1
A 3D model of the Cry j 1 (Fig. 6A) was constructed using the crystal structure of Jun a 1 as the template and the sequence alignment shown in Fig. 5. The overall structure of Cry j 1 is very similar to the crystal structure of Jun a 1. However, there are specific features, such as the location of the glycosylation sites (Figs. 5 and 6A) that are quite different in the two proteins. As indicated by the surface plots of the Cry j 1 model (Fig. 6B), the surface exposure of all four epitope regions are similar to those of Jun a 1. This observation was confirmed quantitatively, using our GETAREA program (http://www.scsb.utmb.edu/cgi-bin/getaform.tcl) (data not shown).
Fig. 6.

Model structure of Cry j 1 (A) Ribbon diagram (front and back views) showing the epitopic regions previously defined for Jun a 1 and colored: 1: aa 71–83, pink; 2: aa 218–230, coral; 3: aa 231–237, purple; 4: aa 296–308, dark green. The three potential glycosylation sites (residues 137, 170, 272) and proven site (residue 333) are gold. Only glycosylation at residue 333 (arrow) has been confirmed. The three presumed disulfides (7–24, 107–126, 285–291; specified in MPACK according to their linkage in the Jun a 1 crystal structure) are in blue and yellow. (B) Surface plot corresponding to that in A, showing the solvent exposure of the four IgE reactive regions. See A for coloring scheme. (C) Comparison of the exposed surface area of the third epitope and atoms within 7 Å of it (cream colored) in the Cry j 1 model (middle) and in the two molecules in the Jun a 1 crystal structure.
As discussed above, epitope 3 of Jun a 1 was not recognized by Japanese patient sera, even though it has the same sequence in both Jun a 1 and Cry j 1. We thus compared the surface exposure of the residues that define epitope 3 of Jun a 1 and the atoms within 7 Å of these residues in the Cry j 1 model with those in the Jun a 1 crystal structure. The surface exposure of epitope 3 varies between the two molecules in the crystal structure of Jun a 1 (Fig. 6C), due to a slightly different position of Lys211 in the two molecules in the unit cell. Our model of Cry j 1 is based on the A conformer, which is less exposed. As Lys211 and most of the other residues within 7 Å of epitope 3 residues are identical in Cry j 1 and Jun a 1, we looked for how the single alteration (Ala266 is Cys in Cry j 1) in the sequence of epitope 2 could affect epitope 3. Although Cys266 in Cry j 1, like Cys171 in Jun a 1, is not near any other cysteine in the molecule, the Sγ of Cys226 lies within 4 Å of the hydrophobic side chains of Phe193, Phe222, Val198 and Met230. Interaction between Cys266 and other residues in this group could affect the relative surface exposure of the epitope 3 area of Cry j 1, and might also influence catalytic activity.
4. Discussion
Cry j 1 was first identified and characterized by Yasueda et al. (1983). A single IgE epitope was identified near the C-terminus (Asn331-Arg352; Taniai et al., 1993). Recognition of this epitope by patient IgE was shown to require glycosylation. However, since none of the five known allergenic homologues of Cry j 1 share this glycosylation site (Midoro-Horiuti et al., 2001; Aceituno et al., 2000; Suzuki et al., 1996; Sone et al., 1994), this epitope cannot be shared with the other known cedar allergens (Midoro-Horiuti et al., 1992; Schwietz et al., 2000). The presence of additional IgE epitopes on Cry j 1 was demonstrated by the finding that three unique groups of anti-Cry j 1 mAbs inhibited the binding to Cry j 1 of IgE from the sera of patients with Japanese cedar hypersensitivity (Sakaguchi et al., 1997). However, these epitopes have not been localized to specific structural elements or regions of Cry j 1.
4.1. Patients sensitized to Japanese cedars recognize three of four linear Jun a 1 epitopes
The goal of the present study was to use our current understanding of the structure of Jun a 1 and its IgE epitopes to identify structurally similar regions in Cry j 1 and determine the extent to which these are responsible for the cross-reactivity between Jun a 1, Cry j 1 and probably other group 1 cedar allergens. The sera from 13 out of 17 (76.5%) Japanese patients which contained IgE reactive to Cry j 1, demonstrated cross-reactivity to mountain cedar pollen extracts by ImmunoCAP. Regression analysis indicated that approximately 30% of the variability of IgE binding to Japanese cedar pollen could be explained by the extent of their binding to mountain cedar pollen (r2 = 0.35, Fig. 1). The role of the group 1 allergens in this cross-reactivity was indicated by inhibition assays, in which the binding of IgE from Japanese cedar hypersensitivity patients was inhibited by this purified Jun a 1 and Cry j 1 (Fig. 2). The extent of inhibition suggested that about half of the IgE reactivity to Japanese cedar pollen was directed against Cry j 1 and approximately 30% of that anti-Cry j 1 reactivity could be inhibited by Jun a 1. Thus, the regression analysis of the direct and cross-reactivity and direct and cross-inhibition experiments both suggest that approximately 30% of the serum IgE anti-Japanese cedar pollen antibodies and the anti-Cry j 1 antibodies cross-react with mountain cedar pollen and Jun a 1, respectively. We conclude that antibodies against Cry j 1 contribute to the cross-reactivity between Japanese cedar and mountain cedar pollen.
We have previously shown that two of the major IgE epitopes of Jun a 1 (2 and 3) are located near the highly conserved pectate lyase catalytic site (Fig. 5; Midoro-Horiuti et al., 2003) and postulated that antibodies directed against those epitopes might cross-react extensively within group 1 cedar allergens. In fact, IgE from patients with Japanese cedar pollen hypersensitivity did react with synthetic peptides representing epitope 2 of Jun a 1. We also found that these patients produced IgE antibodies that reacted with peptides representing two other Jun a 1 epitopes (1 and 4). Thus, the Japanese cedar-sensitive patients recognize three of the four linear epitopes we have described for Jun a 1. However, despite the sequence identity and structural similarity between the epitope 3 regions of Jun a 1 and Cry j 1, the Japanese cedar patients we tested did not have IgE antibodies that reacted with the peptides that represent Jun a 1 epitope 3.
4.2. Structural similarity between IgE epitopes of Jun a 1 and Cry j 1
A 3D model of Cry j 1, based on the crystal structure of Jun a 1, suggested a structural basis for the sharing of the linear epitopes of Jun a 1 and Cry j 1. In keeping with their high sequence identity, the RMSD between the template and the model was quite low (0.39 Å), and the solvent exposure of all four epitope areas was quite similar. However, fine details of the surface could not be fully discriminated between shared and unique epitopes. Cross-reactivity of epitopes in closely related allergenic proteins from different species is a function both of the sequence and the structural context (Beardslee et al., 2000; Ferreira et al., 1998; Jenkins et al., 2005). For example, although soybean glycinin and that of peanut share IgE binding regions, alanine scanning of the peptides indicates that the structures recognized by IgE antibodies are probably different in these two proteins (Beardslee et al., 2000).
Similarly, subtle differences in the less ordered exterior loops and side chain between the two cedar pollen allergens could account for the lack of immune responses to epitope 3 in Cry j 1-sensitized patients. In keeping with this suggestion, there are slight differences in the solvent exposure of residues in the epitope 3 region between the two Jun a 1 molecules in the crystallographic unit cell (Fig. 6B and C). This suggests that within the ensemble of Jun a 1 structures in solution, there may be a subset of Jun a 1 molecules which permit sufficient exposure of the epitope 3 region for IgE binding. The ensemble of Cry j 1 structures may be more biased toward the non-reactive state. Another possibility is that alterations in the epitope 2 sequence could affect the overall exposure of the epitope 3 of Cry j 1 (Fig. 6D).
Alternatively, environmental alteration of Cry j 1, such as binding of an enzyme substrate or inhibitor near the pectin lyase-like active site might reduce the immunogenicity of the epitope 3 region. This concept is in keeping with the observation that Cry j 1 has been shown to have some pectate lyase activity (Taniguchi et al., 1995), while we have been unable to demonstrate any pectinolytic activity of purified Jun a 1 (data not shown). Another possible explanation for the differences in immunologic recognition may be the proximity of a T-cell epitope with the B-cell epitope 3. Genetic differences in response to this T-cell epitope could alter the IgE responses to this region.
The analysis of the responses to the epitope 4 region also gave unexpected results. The Jun a 1 13-mer peptide Trp296-Tyr308 was recognized by IgE antibodies from Japanese cedar-sensitive patients, despite the three nonconserved amino acid differences between Cry j 1 and Jun a 1. However, the detailed analysis for this area with 13-mer peptides, which differed by two residues demonstrated that the Japanese cedar-sensitive patients recognized only one of the three peptides recognized by mountain cedar sensitive patients. Taken together these finding suggest the IgE response to this region is driven by a structure that is conserved despite extensive amino acid substitution.
4.3. MAbs to Cry j 1 define additional shared and unique epitopes
The results of our cross-reactivity and inhibition experiments indicate that Cry j 1 also displays one or more epitopes that are not present on Jun a 1. In an attempt to map or characterize the structure of these unique epitopes, we tested the reactivity of a group of anti-Cry j 1 mAb with Jun a 1 and its synthetic peptides (Table 1).
One of these mAbs (S95) inhibits the binding of IgE antibodies in sera from 10 of 21 Japanese cedar-sensitive patients (Sakaguchi et al., 1997) to Cry j 1. In the experiments described here, S95 also interfered with the binding of IgE antibodies from sera of mountain cedar-sensitive patients to Jun a 1. This suggests that the Cry j 1 EP-5 epitope, described by Sakaguchi et al. is the same as our Jun a 1 epitope 2. Another major IgE epitope of Cry j 1, identified by mAb S131 (EP-3) binds to Jun 1 and inhibits the binding of Texas sera. However, S131 did not react with any of the synthetic peptides representing Jun a 1, suggesting that S131 identifies a conformational or discontinuous epitope shared by Cry j 1 and Jun a 1. Finally, one group of mouse mAbs that identify EP-1 (Sakaguchi et al., 1997) only reacts with Cry j 1. It is possible that this epitope is formed by the glycopeptide structure described by Taniai et al. (1993). The absence of this N-glycosylation site in all of the other group 1 cedar allergens might explain the unique nature of this Cry j 1 epitope. None of the mouse mAbs we tested reacted with peptides representing Jun a 1 epitopes 1, 3 or 4 (all recognized by human IgE anti-Cry j 1).
In summary, the current study provides the first evidence for Cry j 1 epitopes that are homologous to Jun a 1 epitopes 1 and 4 and maps Cry j 1 epitope EP-5 to a genetically conserved region of that allergen. Thus, our findings suggest that structurally similar regions are prominent components of the humoral immune response to group 1 allergens in genetically distinct populations of humans. However, both of the homologous allergens we examined also display distinct epitopes. In fact, our cross-reactivity and cross-inhibition experiments suggest that the majority of anti-Cry j 1 IgE antibodies do not cross-react with Jun a 1 linear epitopes. Some of these anti-Cry j 1 antibodies probably react with a glycopeptide epitope(s) that are absent from other group 1 allergens. Interestingly, the one epitope that did not cross-react has an identical sequence in all of the group 1 cedar allergens. It is not clear why this region of Cry j 1 does not induce the production of antibodies that cross-react with Jun a 1, although analysis of that region suggests that this epitope might be somewhat less exposed region in Cry j 1 than in some Jun a 1 molecules. Resolving the crystal structure of Cry j 1 and mutagenesis of selected regions of these molecules will be required to fully explain the shared and unique epitopes of these highly homologous allergens.
The approach we used in the current study demonstrates the value and limitations of using amino acid sequence identity, mAbs and 3D homology modeling to define new epitopes and to characterize the structural basis of epitope sharing. Identifying shared and unique epitopes may have both fundamental and clinical value. Understanding the structural requirements for the IgE responses and reactions should provide a basis for identifying the allergenic potential of new proteins, thereby preventing their introduction into the environment or reducing the extent of contact by humans. In addition, development of new generations of immunologic reagents for allergy testing and specific immunotherapy could be expedited if well-characterized reagents could be used in groups of patients who are allergic to homologous allergens.
Acknowledgments
We wish to thank Dr. Julius van Bavel for providing sera from patient with mountain cedar hypersensitivity. This work was supported by a NICHD-sponsored Child Health Research Center (P30 HD27841, New Project Award, T.M.H.), NIEHS Center for Environmental Science (ES06676, T.M.H.), the John Sealy Memorial Endowment for Biomedical Research (T.M.H.) from UTMB, Advanced Technology Program from Texas Higher Education Coordinating Board (0060-2001, R.M.G. and 0036-2003, W.B.), Parker B. Francis Fellowship in Pulmonary Research from Francis Families Foundation (T.M.H.), R01AI052428 (R.M.G.), K08 AI055792 (T.M.H.) and FDA (002249, W.B.).
Abbreviations
- Cry j 1
group 1 allergen of Japanese cedar (Cryptomeria japonica)
- Jun a 1
group 1 allergen of mountain cedar (Juniperus ashei)
- mAb
monoclonal antibody
- MW
molecular weight
- PDB
protein data bank
- PL
pectate lyase
- RMSD
root-mean-square deviation
- 3D
three-dimensional
References
- Aceituno E, Del PV, Minguez A, Arrieta I, Cortegano I, Cardaba B, Gallardo S, Rojo M, Palomino P, Lahoz C. Molecular cloning of major allergen from Cupressus arizonica pollen: Cup a 1. Clin Exp Allergy. 2000;30:1750–1758. doi: 10.1046/j.1365-2222.2000.00949.x. [DOI] [PubMed] [Google Scholar]
- Arilla MC, Ibarrola I, Martinez A, Asturias JA. Quantification assay for the major allergen of Cupressus sempervirens pollen, Cup s 1, by sandwich ELISA. Allergol Immunopathol (Madr) 2004;32:319–325. doi: 10.1016/s0301-0546(04)79263-0. [DOI] [PubMed] [Google Scholar]
- Beardslee TA, Zeece MG, Sarath G, Markwell JP. Soybean glycinin G1 acidic chain shares IgE epitopes with peanut allergen Ara h 3. Int Arch Allergy Immunol. 2000;123:299–307. doi: 10.1159/000053642. [DOI] [PubMed] [Google Scholar]
- Czerwinski EW, Midoro-Horiuti T, White MA, Brooks EG, Goldblum RM. Crystal structure of Jun a 1, the Major Cedar pollen allergen from Juniperus ashei, reveals pararel beta-helical core. J Biol Chem. 2005;280:3740–3746. doi: 10.1074/jbc.M409655200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira F, Ebner C, Kramer B, Casari G, Briza P, Kungl AJ, Grimm R, Jahn-Schmid B, Breiteneder H, Kraft D, Breitenbach M, Rheinberger HJ, Scheiner O. Modulation of IgE reactivity of allergens by site-directed mutagenesis: potential use of hypoallergenic variants for immunotherapy. FASEB J. 1998;12:231–242. doi: 10.1096/fasebj.12.2.231. [DOI] [PubMed] [Google Scholar]
- Fraczkiewicz R, Braun W. Exact and efficient analytical calculation of the accessible surface areas and their gradients for macromolecules. J Comput Chem. 1998;19:319–333. [Google Scholar]
- Ivanciuc O, Mathura V, Midoro-Horiuti T, Braun W, Goldblum RM, Schein CH. Detecting potential IgE-reactive sites on food proteins using a sequence and structure database, SDAP-Food. J Agric Food Chem. 2003;51:4830–4837. doi: 10.1021/jf034218r. [DOI] [PubMed] [Google Scholar]
- Ivanciuc O, Oezguen N, Mathura VS, Schein CH, Xu Y, Braun W. Using property based sequence motifs and 3D modeling to determine structure and functional regions of proteins. Curr Med Chem. 2004;11:583–593. doi: 10.2174/0929867043455819. [DOI] [PubMed] [Google Scholar]
- Ivanciuc O, Schein CH, Braun W. Data mining of sequences and 3D structures of allergenic proteins. Bioinformatics. 2002;18:1358–1364. doi: 10.1093/bioinformatics/18.10.1358. [DOI] [PubMed] [Google Scholar]
- Jenkins JA, Griffiths-Jones S, Shewry PR, Breiteneder H, Mills EN. Structural relatedness of plant food allergens with specific reference to cross-reactive allergens: an in silico analysis. J Allergy Clin Immunol. 2005;115:163–170. doi: 10.1016/j.jaci.2004.10.026. [DOI] [PubMed] [Google Scholar]
- Koradi R, Billeter M, Wuthrich K. MOLMOL: a program for display and analysis of macromolecular structures. J Mol Graph. 1996;14:51–32. doi: 10.1016/0263-7855(96)00009-4. [DOI] [PubMed] [Google Scholar]
- Midoro-Horiuti T, Goldblum RM, Brooks EG. Identification of mutation in the genes for the pollen allergens of eastern red cedar (Juniperus virginiana) Clin Exp Allergy. 2001;31:771–778. doi: 10.1046/j.1365-2222.2001.01079.x. [DOI] [PubMed] [Google Scholar]
- Midoro-Horiuti T, Goldblum RM, Kurosky A, Goetz DW, Brooks EG. Isolation and characterization of the mountain cedar (Juniperus ashei) pollen major allergen Jun a 1. J Allergy Clin Immunol. 1999;104:608–612. doi: 10.1016/s0091-6749(99)70331-3. [DOI] [PubMed] [Google Scholar]
- Midoro-Horiuti T, Mathura V, Schein CH, Braun W, Yu S, Watanabe M, Lee JC, Brooks EG, Goldblum RM. Major linear IgE epitopes of mountain cedar pollen allergen Jun a 1 map to the pectate lyase catalytic site. Mol Immunol. 2003;40(8):555–562. doi: 10.1016/s0161-5890(03)00168-8. [DOI] [PubMed] [Google Scholar]
- Midoro-Horiuti T, Nouno S, Seino Y. Skin tests of pollen grains of Taxodiaceae and Cupressaceae in children with bronchial asthma. Acta Paediatr Jpn. 1992;34:501–504. doi: 10.1111/j.1442-200x.1992.tb00996.x. [DOI] [PubMed] [Google Scholar]
- Murtazina D, Puchkaev AV, Schein CH, Oezguen N, Braun W, Nanavati A, Pikuleva IA. Membrane-protein interactions contribute to efficient 27-hydroxylation of cholesterol by mitochondrial cytochrome P450 27A1. J Biol Chem. 2002;277:37582–37589. doi: 10.1074/jbc.M204909200. [DOI] [PubMed] [Google Scholar]
- Sakaguchi M, Hashimoto M, Nigi H, Yasueda H, Takahashi Y, Watanabe M, Nagoya T, Taniguchi Y, Kurimoto M, Inouye S. Epitope specificity of IgE antibodies to a major allergen (Cry j 1) of Japanese cedar pollen in sera of humans and monkeys with pollinosis. Immunology. 1997;91:161–166. doi: 10.1046/j.1365-2567.1997.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein CH, Nagle GT, Page JS, Sweedler JV, Xu Y, Painter SD, Braun W. Aplysia attract biophysical characterization modeling of a water-borne pheromone. Biophys J. 2001;81:463–472. doi: 10.1016/S0006-3495(01)75714-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwietz LA, Goetz DW, Whisman BA, Reid MJ. Cross-reactivity among conifer pollens. Ann Allergy, Asthma Immunol. 2000;84:87–93. doi: 10.1016/S1081-1206(10)62746-9. [DOI] [PubMed] [Google Scholar]
- Soman KV, Midoro-Horiuti T, Ferron JC, Goldblum RM, Brooks EG, Kurosky A, Braun W, Schein CH. Homology modeling and characterization of IgE binding epitopes of mountain cedar allergen Jun a 3. Biophys J. 2000;79:1601–1609. doi: 10.1016/S0006-3495(00)76410-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soman KV, Schein CH, Zhu H, Braun W. Homology modeling and simulations of nuclease structures. In: Schein CH, editor. Methods in Molecular Biology. Vol. 160. Humana press; Totowa, NJ: 2001. pp. 263–281. [DOI] [PubMed] [Google Scholar]
- Sone T, Komiyama N, Shimizu K, Kusakabe T, Morikubo K, Kino K. Cloning and sequencing of cDNA coding for Cry j I, a major allergen of Japanese cedar pollen. Biochem Biophys Res Commun. 1994;199:619–625. doi: 10.1006/bbrc.1994.1273. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Komiyama N, Itoh M, Itoh H, Sone T, Kino K, Takagi I, Ohta N. Purification, characterization and molecular cloning of Cha o 1, a major allergen of Chamaecyparis obtusa (Japanese cypress) pollen. Mol Immunol. 1996;33:451–460. doi: 10.1016/0161-5890(95)00147-6. [DOI] [PubMed] [Google Scholar]
- Taniai M, Kayano T, Takakura R, Yamamoto S, Usui M, Ando S, Kurimoto M, Panzani R, Matuhasi T. Epitopes on Cry j I and Cry j II for the human IgE antibodies cross-reactive between Cupressus sempervirens and Cryptomeria japonica pollen. Mol Immunol. 1993;30:183–189. doi: 10.1016/0161-5890(93)90090-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi Y, Ono A, Sawatani M, Nanba M, Kohno K, Usui M, Kurimoto M, Matuhasi T. Cry j I, a major allergen of Japanese cedar pollen, has pectate lyase enzyme activity. Allergy: Eur J Allergy Clin Immunol. 1995;50:90–93. doi: 10.1111/j.1398-9995.1995.tb02489.x. [DOI] [PubMed] [Google Scholar]
- Yasueda H, Yui Y, Shimizu T, Shida T. Isolation and partial characterization of the major allergen from Japanese cedar (Cryptomeria japonica) pollen. J Allergy Clin Immunol. 1983;71:77. doi: 10.1016/0091-6749(83)90550-x. [DOI] [PubMed] [Google Scholar]