Abstract
This paper utilizes cyclodextrin-based host—guest chemistry in a microfluidic device to modulate the crystallization of membrane proteins and the process of concentration of membrane protein samples. Methyl-β-cyclodextrin (MBCD) can efficiently capture a wide variety of detergents commonly used for the stabilization of membrane proteins by sequestering detergent monomers. Reaction Center (RC) from Blastochloris viridis was used here as a model system. In the process of concentrating membrane protein samples, MBCD was shown to break up free detergent micelles and prevent them from being concentrated. The addition of an optimal amount of MBCD to the RC sample captured loosely bound detergent from the protein-detergent complex and improved sample homogeneity, as characterized by dynamic light scattering. Using plug-based microfluidics, RC crystals were grown in the presence of MBCD, giving a different morphology and space group than crystals grown without MBCD. The crystal structure of RC crystallized in the presence of MBCD was consistent with the changes in packing and crystal contacts hypothesized for removal of loosely bound detergent. The incorporation of MBCD into a plug-based microfluidic crystallization method allows efficient use of limited membrane protein sample by reducing the amount of protein required and combining sparse matrix screening and optimization in one experiment. The use of MBCD for detergent capture can be expanded to develop cyclodextrin-derived molecules for fine-tuned detergent capture and thus modulate membrane protein crystallization in an even more controllable way.
1. Introduction
This paper describes the use of cyclodextrin-based host—guest chemistry in a microfluidic device to control crystallization of membrane proteins. Preparation of diffraction quality crystals is a major barrier to obtaining structures of membrane proteins, which is critical to both fundamental and applied molecular sciences.1 Most crystallization methods rely on detergents to solubilize membrane proteins and to maintain protein stability and activity;2,3 this use of detergents leads to formation of crystals of the protein-detergent complex (PDC). Furthermore, in most crystallization methods, excess detergent above the critical micelle concentration (CMC) is often required to solubilize the protein or is obtained as an artifact of the procedures used to concentrate the protein sample for crystallization. Excess detergent leads to the formation of free micelles that increase the heterogeneity of the sample and affect both nucleation and growth of crystals.4-6 Excess detergent also leads to loosely bound detergent molecules in the PDC that may interfere with crystal contacts.7,8 Bulk polymeric materials have been used to facilitate extraction of detergent from samples of membrane proteins,3 but such materials carry the risk of binding the protein in addition to detergent. We hypothesized that carefully controlled in situ removal of detergent at the molecular level during crystallization could be used to modulate crystallization of membrane proteins by three different means: (i) improving homogeneity of the sample, (ii) breaking up free micelles, and (iii) capturing loosely bound detergent from the PDC, thus increasing the accessible surface area of the protein for crystal contacts (Figure 1A).
Figure 1.
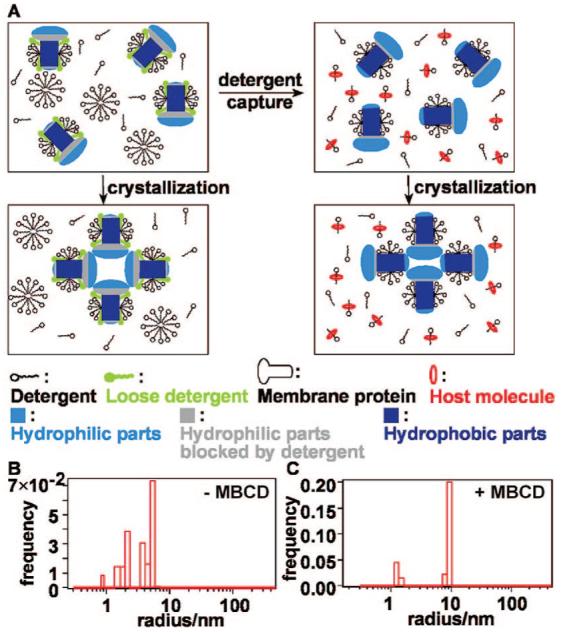
(A) Schematic representation of the proposed effects of detergent capture on crystallization of membrane proteins. Removal of the loosely bound detergent and free micelles improves sample homogeneity and available crystal contacts. (B) DLS characterization of the starting RC samples showed the heterogeneity in size of PDC and free detergent micelles. (C) DLS revealed improved homogeneity after capture of LDAO detergent with MBCD.
In this paper, we validated these ideas by using methyl-β-cyclodextrin (MBCD) to capture detergent from solutions of membrane proteins during both concentration and crystallization steps. Cyclodextrins make up of a family of cyclic oligosaccharides. Typical cyclodextrins contain six to eight glucose units in a ring, creating a truncated cone shape and forming a hydrophobic cavity inside the ring.9 They bind hydrophobic or amphiphilic guest molecules, such as detergents, inside the cavity and are therefore unlikely to bind to hydrophobic surface patches of a folded membrane protein.10,11 Binding of detergents by cyclodextrins has been used in a variety of applications in the context of membrane proteins, including the stimulation of the exchange of PDCs into lipid bilayers11 and the structural studies of two-dimensional crystals by electron microscopy,10 but it has not been tested previously in three-dimensional crystallization of membrane proteins. We performed crystallization experiments by using a plug-based microfluidic hybrid method for protein crystallization to accelerate and simplify the process.12,13 Reaction Center (RC) from Blastochloris viridis was chosen as a model protein.
2. Experimental Section
Chemicals and Materials
All chemicals purchased from commercial sources were used as received unless otherwise stated. The details on chemicals and instruments are provided in the Supporting Information.
Fabrication of PDMS Devices
All microfluidic devices were fabricated from poly(dimethylsiloxane) (PDMS). Microchannels with rectangular cross sections were fabricated with rapid prototyping.14 The channel walls were functionalized with (tridecafluoro-1,1,2,2-tetrahydrooctyl)-1-trichlorosilane to render them hydrophobic and fluorophilic.15
Dynamic Light Scattering (DLS) Measurement to Characterize Stoichiometric Ratio of MBCD/LDAO Complex Formation
DLS was performed at room temperature on a Precision Detectors Inc. PD2000 light scattering instrument at 800 nm with a scattering angle of 90°. Detailed preparation of samples and parameters used for measurements are provided in the Supporting Information.
1HNMR Titration Analysis to Characterize the Formation of MBCD/LDAO Complex
To further characterize the formation of the MBCD/LDAO complex, 1HNMR titration analysis was performed by using D2O as a solvent. Sodium 3-trimethylsilylpropionate at 4 mM was used as an internal standard. To identify chemical shifts from MBCD, samples of 1 mM, 2 mM, and 6 mM MBCD were measured with 1HNMR. To identify chemical shifts from LDAO, a sample of 2 mM LDAO was measured. For the titration analysis, the LDAO concentration was kept at 2 mM, while MBCD concentrations were tested at 0.5, 1, 2, 3, 4, and 8 mM, yielding MBCD:LDAO ratios of 1:4, 1:2, 1:1, 1.5:1, 2:1, and 4:1. The sample of ratio 1.5:1 was remeasured after a 72-hour incubation at room temperature.
Crystallization of RC in the Presence of MBCD or γ-CD
A device with four aqueous inlets plus one oil inlet was used to perform optimization of crystallization of RC. The carrier fluid (oil) was a mixture of perfluoro-tri-n-butylamine and perfluoro-di-nbutylmethylamine (FC-40). The four aqueous streams were (i) precipitant, (ii) buffer, (iii) RC protein, and (iv) an array of 50 nL plugs of MBCD or γ-CD at different concentrations. The composition and concentration of each stream can be found in Supporting Information. The preparation of the array of MBCD or γ -CD plugs was detailed previously.12 All the flow rates were controlled by a Labview subroutine (details in Supporting Information). The trials, in the form of plugs, were transported and stored in Teflon tubing (O.D. = 250 μm and I.D. = 200 μm) which was sealed in glass tubing (O.D. = 3 mm and I.D. = 1.8 mm) that was prefilled with perfluorotripentylamine (FC-70). Crystallization was performed under dimly lit conditions, and the trials were stored in the dark at 23 °C.
Crystal Preparation, X-ray Data Collection, and X-Ray Structure Determination of RC
Cryo-protectant for freezing RC crystals was either paraffin oil or 35% (w/v) glucose, 2.6 M (NH4)2SO4, 4.4 mM LDAO, 0.5% (v/v) TEAP, 1% 1,2.3-hepatanetriol in 50 mM Na2HPO4/NaH2PO4 buffer (pH 6.0). Extraction of crystals of RC grown in the plugs and subsequent mounting is described in the Supporting Information. X-ray diffraction experiments to determine space group for all crystals grown in the presence of MBCD were performed at GM/CA Cat station 23 IDB, BioCars station 14 BM-C and Ls-Cat station 21 ID-D and G of the Advanced Photon Source (Argonne National Laboratory). X-ray diffraction data were processed in HKL2000.16 The new RC trigonal structure was solved by molecular replacement by using PDB 2I5N structure as a starting model and MOLREP17 program in CCP4 suite.18 The rigid-body, positional, and temperature factor refinement was performed by using maximum likelihood target with the program REFMAC5.19 The SigmaA-weighted 2Fobs — Fcalc and Fobs — Fcalc Fourier maps were calculated by using CCP4. The Fourier maps were displayed and examined in TURBO-FRODO20 and COOT.21 The search for new solvent molecules was performed with the help of COOT. The crystal data, data collection, and refinement statistics are summarized in the Supporting Information (Table 2). The coordinates and structure factors have been deposited in the Protein Data Bank with entry code 3D38. Details of crystallographic collection and structure solving are given in the Supporting Information.
Detergent Concentration and Thin Layer Chromatography (TLC)
The preparation of the TLC setup followed a reported procedure.22 Briefly,a 2 L glass beaker was lined with Whatman filter paper and equilibrated with the mobile phase (chloroform/ methanol/ammonium hydroxide, 63:35:5, v/v/v) for 1 h.
TLC Characterization of DDM Concentration
DDM (0.51 mM, 15 mL) in 20 mM Tris (pH = 7.8) and DDM (0.51 mM, 15 mL) in the presence of MBCD (0.51 mM) in 20 mM Tris (pH = 7.8) were concentrated to 650–700 μL. TLC was performed on the detergent solutions pre- and postconcentration through the filter. Six samples were deposited, from left to right: (1) 15 μL sample 1; (2) 5 μL concentrated sample 1; (3) 15 μL solution that passed through the filter from sample 1; (4) 5 μL concentrated sample 2; (5) 5 μL concentrated sample 2; (6) 10 μL solution that passed through the filter from sample 2 (Supporting Information, Figure 6A). The plate was stained with iodine vapor and imaged with a scanner. The DDM spots were analyzed with TotalLab TL100 as described in the Supporting Information. The obtained values were divided into two groups for analysis: lanes 1, 2, 3 and lanes 4, 5, 6. The pixel volumes were first calibrated by volume, and the relative concentration was calculated by defining lanes 1 and 4 as one, for lanes 1, 2, 3 and lanes 4, 5, 6 respectively.
DDM Calibration Curve
To confirm that the pixel volumes on a TLC plate could be linearly correlated to DDM concentrations, a calibration curve was prepared. The range of DDM concentration was from 0 to 50 mM. Full details of the construction for the calibration curve for DDM are given in the Supporting Information.
TLC Characterization of RC and DDM Concentration
Preparation of RC in DDM and the description of the process of concentrating RC samples can be found in the Supporting Information. Five samples were spotted and examined with TLC following the same procedure described above. From left to right: (1) 5 μL mixture of 8.3 mM DDM and 218 mM LDAO; (2) 10 μL unconcentrated sample I; (3) 5 μL sample I; (4) 10 μL unconcentrated sample II; (5) 5 μL concentrated sample II (Supporting Information, Figure 6B). The analysis of the TLC plate was similar to the analysis of the plate in DDM concentration and is detailed in the Supporting Information.
3. Results and Discussion
Stoichiometric Detergent Capture Improved the Homogeneity of the Membrane Protein Sample
We characterized the effect of detergent capture on thehomogeneity of the samples of RC by using DLS. Samples of RC prepared in 3.5 mM of lauryldimethylamine-oxide (LDAO), a standard solution for crystallization,12 showed a heterogeneous mixture of LDAO micelles (hydrodynamic radius, Rh ≈ 1.9 nm) and PDCs (Rh ≈ 4.3 nm) (Figure 1B). Addition of equimolar (3.5 mM) MBCD ≈ dramatically improved the homogeneity of the sample, giving two sharp peaks: one at Rh ≈ 1 nm, assigned to the MBCD/LDAO complex, and another at Rh 8.3 nm, which was about twice as large as PDC of reaction ≈center (Rh ≈ 4.3 nm), and was likely to belong to a RC—RC dimer (Figure 1C). We hypothesized that MBCD captured the detergent molecules that were loosely bound to the protein, exposing the protein interfaces that form the dimer and driving the dimerization by addition of precipitant in the absence of MBCD (Supporting Information, Figure 4). Control DLS experiments (Supporting Information, Figure 1) were consistent with the formation of a 1:1 MBCD:LDAO complex under these conditions. Addition of less than equimolar (insufficient) MBCD to the sample showed that free micelles remained (Supporting Information, Figure 3A). Excess MBCD led to the capture of detergents critical for solubilization and thus led to formation of higherorder aggregates (Supporting Information, Figure 3B). To study the complex formation of MBCD and LDAO, 1HNMR experiments were performed at different MBCD:LDAO ratios (Supporting Information, Figure 2). NMR results were consistent with the formation of a 1:1 MBCD:LDAO complex based on DLS measurements.
Capture of Detergent Changed the Morphology of Crystals of RC
To test whether crystallization could be controlled by the capture of detergent, we set up crystallization trials of RC (experimental details in Supporting Information) in 4.6 mM LDAO in the presence of 4 mM MBCD. In this experiment, 80% (9 out of 11) of the crystals produced in the trial had trigonal morphology (Figure 2A,B). Slightly increasing LDAO concentration (to 5.3 mM) in the trials resulted in mostly tetragonal crystals (5 out of 6). Furthermore, with either no MBCD or insufficient MBCD (2 mM) under the same crystallization conditions, all of the crystals grown had tetragonal morphology (Figure 2C,D). Excess MBCD at 8 mM and 10 mM led to the growth of all trigonal crystals (Supporting Information, Table 1). To test whether the change of morphology was due to the detergent capture, we used γ-cyclodextrin to substitute MBCD. γ-Cyclodextrin does not bind to LDAO.11 We obtained crystals giving the same tetragonal morphology (Figure 2E,F) as those with no MBCD present (Supporting Information, Table 1). 1,2,3-Heptanetriol, a small amphiphilic molecule, was the additive in all of the crystallization trials, but it was not expected to interfere with the binding of detergent to MBCD because the shorter alkyl chain (C4) has much lower affinity toward -β cyclodextrin molecules, and thus 1,2,3-heptanetriol (C4) will not compete with the detergent (C12) for MBCD.23
Figure 2.
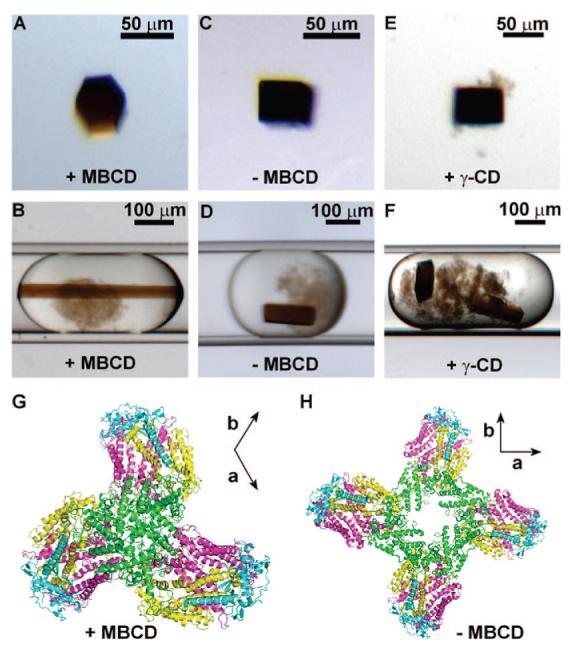
MBCD captures free and loosely bound detergent and changes the morphology and packing of RC crystals. (A) Trigonal crystal grown in the presence of 4 mM MBCD and 4.6 mM LDAO. The crystal is shown in a 2 μL cryo-protectant droplet.(B) Trigonal crystal in a microfluidic plug. (C) Tetragonal crystal grown at 44 mM LDAO (∼40 CMC). The crystal is shown in a 2μL cryo-protectant droplet. (D) Tetragonal crystal in a microfluidic plug. (E) Tetragonal crystal grown in the presence of 8 mM γ-CD and 4.6 mM LDAO. The crystal is shown in a 2 μL cryo-protectant droplet. (F) Tetragonal crystals in a plug containing 8 mM γ-CD. (G) The arrangement of RC proteins in trigonal crystals P3121, a = b = 241.2 Å, c = 113.4 Å. (H) The arrangement of RC proteins in tetragonal crystals P43212 a = b = 220.4 Å, c = 113.0 Å. (G) and (H) are both viewed along the c axis (31-fold for C and 43-fold for F). Axes a and b in the lattices are shown on the top right of each figure. Green: subunit C; Cyan: subunit H; yellow: subunit M; and pink: subunit L.
Protein Contacts Closer to Membrane Planes Were Obtained in Trigonal Crystals as Compared to Tetragonal Crystals
To investigate how crystal packing and crystal contacts changed in trigonal crystals, as compared to tetragonal crystals, we used X-ray diffraction to solve the crystal structure at 3.2 Å resolution (PDB ID: 3D38). The trigonal crystals displayed high solvent content (∼80%); the observed X-ray resolution was consistent with the expected resolution for this amount of solvent in the crystal.24 We did not find any electron density that could be assigned to MBCD, indicating MBCD was unlikely to be responsible for mediating and changing the crystal contacts, but we cannot rigorously exclude the possibility that presence of MBCD somehow negatively affected diffraction. We attribute the change of the space group upon addition of MBCD to its role in detergent capture, rather than to binding of MBCD within the crystal structure.
Despite the higher solvent content in trigonal crystals (∼80% versus ∼70% found in the tetragonal structure), we observed significant shrinkage of the solvent channel shaped by the cytochrome C subunit of RC around the shortest axis, c,ofthe trigonal crystal, (Figure 2G) as compared to the solvent channel around the tetragonal crystals (Figure 2H). The new contacts formed by the cytochrome C subunit in the trigonal crystals were closer to the membrane plane and had more contacting areas than those contacts formed by cytochrome C subunit in the tetragonal crystals (Supporting Information, Figure 4). This observation supported the hypothesis that more residues, especially the ones near the membrane plane, should have been exposed to form crystal contacts as loosely bound detergents were removed. Furthermore, the residues mediating the formation of RC dimer were identical in both trigonal and tetragonal crystals (Supporting Information, Figure 4), and thus did not account for the change of the unit cell from tetragonal to trigonal.
Detergent Capture Minimized Concentrating Detergent in the Process of Concentration of Membrane Proteins
We tested whether MBCD could also be used during protein concentration to remove excess detergent. During any concentration step in the preparation of membrane proteins, solubilizing detergent in the sample is subject to concentration because of the presence of free micelles that cannot pass through the cutoff filter (Figure 3A,B). To address this problem, we prepared a sample of 0.51 mM (∼3 CMC) n-dodecyl-β-maltopyranoside (DDM). DDM was chosen because it forms large micelles8 and is believed to be especially problematic during the concentrating process. Samples with and without equimolar (0.51 mM) MBCD were concentrated through a 30 kDa cutoff filter. In both cases, the starting volume was 15 mL and the final volume after concentrating was ∼700 μL. The relative concentrations after DDM before and passing through the filter were determined by using thin layer chromatography (TLC, Supporting Information, Figure 6A).22 Running DDM samples with different concentrations on the TLC plate confirmed that the concentrations could be linearly correlated to the intensity of the deposits (Supporting Information, Figure 5). Without MBCD, the detergent sample was concentrated 18-fold; whereas, with MBCD present, the detergent sample was only concentrated 1.2 fold (Figure 3C). When concentrating the pure DDM solution, DDM micelles (∼60 kDa8) could not pass through the 30 kDa filter and thus became concentrated. However, when equimolar MBCD was added, MBCD broke down the micelles and formed a host—guest complex with monomeric DDM. This complex could easily pass through the filter. Using RC, we confirmed that the presence of MBCD did not interfere with the process of concentrating the protein (Supporting Information, Figures 6B and 7) and RC maintained intact according to UV—visible spectrometry (Supporting Information, Figure 8).
Figure 3.
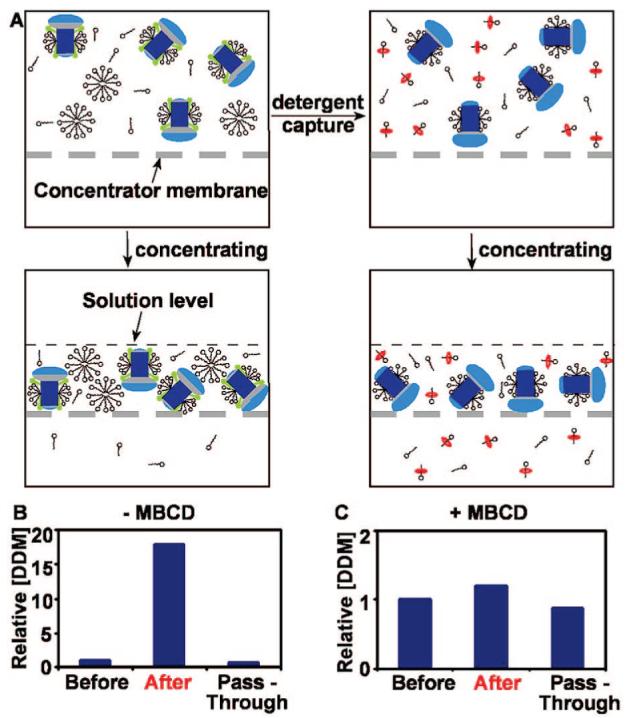
Using MBCD to minimize the concentration of detergent micelles in the process of concentrating membrane proteins. (A) Left column shows that during concentration of membrane protein samples, free micelles are also concentrated; right column shows that during concentration, free detergent does not become concentrated because MBCD captures loosely bound and micellar detergent. (B),(C) Concentrating DDM solutions without (B) and with (C) MBCD. “Before” denotes the concentration of DDM before passing through a 30 kDa filter, the sample volume was 15 mL; “After” denotes DDM concentration retained above the filter, the sample volume was ∼700 μL; and “Pass-Through” denotes the concentration of the solution that passed through the filter. (B) Relative DDM concentration before and after passing through a 30 kDa cutoff filter in the absence of MBCD. DDM micelles could not pass through this filter and were retained in solution. (C) Addition of equimolar MBCD to DDM allowed DDM monomers to pass through the filter and reduced the concentration of DDM remaining in solution.
4. Conclusions
We conclude with three remarks. First, the removal of detergents with methyl-β-cyclodextrin (MBCD) should not be limited only to LDAO and DDM reported in this paper. Different types of detergents with alkyl chains are all expected to form complexes with MBCD at high affinity, and thus the detergent capture should be applicable.10,11 Furthermore, the method reported in this paper also is not limited to the use of MBCD as a host molecule. α- And γ-cyclodextrins bear a smaller and a larger cavity, respectively. For amphiphilic molecules with shorter alkyl chains (<C8), α-cyclodextrin is a better candidate to be a capturing host,23 while γ- cyclodextrin is suggested for use toward detergents with bulky chains such as CHAPS and cholate.11 The specific combination of detergent and cyclodextrin may allow manipulation of different detergents, and even lipids existing in protein sample, by selectively removing detergents but not native lipids. Alternatively, one may use this approach to capture selectively the detergent that could interfere with crystallization (such as those optimal for purification) while not removing a detergent optimal for crystallization.
Second, methyl-β-cyclodextrin (MBCD) is an inexpensive and widely available reagent that can be immediately incorporated into homemade and commercial crystallization screens to remove most of the detergents used during crystallization. Such experiments would be able to rapidly address the remaining questions: are results reported here general, and for what types of membrane proteins are they most applicable? Is there a general correlation between removal of detergent and change in solvent content of the crystal? Is MBCD inert to the majority of membrane proteins? Here we used MBCD in the plug-based25 hybrid method,12 but it should also be applicable in the context of both traditional and miniaturized crystallization methods.26,27 MBCD may also be used to remove excess detergent by breaking up detergent micelles during concentrating, or to remove excess detergent in exchange for designer detergents that do not bind MBCD.
Third, host—guest chemistry is an advanced field and can be used to fine-tune the processes of removing detergent molecules in a multitude of ways, one example being the addition of judicious amounts of detergent-removing molecules. In this paper, we showed that both insufficient and excess addition had negative effects (Supporting Information, Figure 3), although a very precise ratio of MBCD to detergent was not needed, with an optimal range from 4:5.3 to 10:4.6. An improvement to this method that may facilitate further developments in crystallization is the activation of the host with more controlled kinetics. MBCD appears to remove the free detergent micelles and also to remove some of the loosely bound detergent, leading to new crystal contacts through residues closer to the membrane plane. However, removing more detergent too rapidly would probably induce precipitation of the protein. In contrast, time-controlled removal of loosely bound detergent may lead to nucleation and more orderly growth of the protein crystal. This could be achieved with a host that forms over time under the crystallization conditions or with a self-inhibited host that spontaneously loses the inhibitor over time. Potential improvement also lies in the synthesis of hosts with more controlled thermodynamics suitable for selective binding of and discrimination among different bound forms of a detergent or between traditional and designer3 detergents. Various small molecules, such as sterols or lipids, may be critical to a protein’s crystallizability and function. This host-guest chemistry approach presents a risk of removing critical small molecules, but it also offers an opportunity for researchers to develop hosts for selectively removing detergents, but not native small molecules, or for delivering those small molecules to membrane proteins.
Supplementary Material
Acknowledgment
This work was supported in part through the NIH Roadmap for Medical Research (R01 GM075827-01) and UC/ANL Collaborative Seed Funding. We thank ATCG3D funded by the NIGMS and NCRR under the PSI-2 Specialized Center program (U54 GM074961) for providing some of the equipment used in this work. V.T. was supported by ATCG3D. A.M.S. was supported by an EPSRC LSI Postdoctoral Fellowship. S.N. was supported by the NIH Roadmap Physical and Chemical Biology undergraduate training program at UC. We would like to thank Eva Chi for discussion about DLS experiments and Elizabeth W. Boyd for contributions in writing and editing this manuscript. Use of the Argonne National Laboratory LS-CAT beamlines, BioCARS beamlines, and GM/CA beamlines at the Advanced Photon Source was supported by the U.S. Department of Energy, Basic Energy Sciences, Office of Science, under Contract No. DE-AC02-06CH11357. GM/CA CAT has been funded in whole or in part with Federal funds from the National Cancer Institute (Y1-CO-1020) and the National Institute of General Medical Science (Y1-GM-1104). Use of the BioCARS Sector 14 was supported by the National Institutes of Health, National Center for Research Resources, under Grant Number RR07707. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor for the support of this research program (Grant 085P1000817).
Footnotes
Supporting Information Available: Details of chemicals and equipment used, experimental details for the preparation of samples for DLS and TLC, the details of the Labview program used to control the microfluidic crystallization method, and results obtained for control experiments and the construction of calibration curves. This material is available free of charge via the Internet at http://pubs.acs.org/.
References
- (1).Ostermeier C, Michel H. Curr. Opin. Struct. Biol. 1997;7:697–701. doi: 10.1016/s0959-440x(97)80080-2. [DOI] [PubMed] [Google Scholar]
- (2).Zhang QH, Ma XQ, Ward A, Hong WX, Jaakola VP, Stevens RC, Finn MG, Chang G. Angew. Chem., Int. Ed. 2007;46:7023–7025. doi: 10.1002/anie.200701556. [DOI] [PubMed] [Google Scholar]
- (3).Seddon AM, Curnow P, Booth PJ. Biochim. Biophys. Acta: Biomembr. 2004;1666:105–117. doi: 10.1016/j.bbamem.2004.04.011. [DOI] [PubMed] [Google Scholar]
- (4).Wiener MC. Methods. 2004;34:364–372. doi: 10.1016/j.ymeth.2004.03.025. [DOI] [PubMed] [Google Scholar]
- (5).Wiener MC, Snook CF. J. Cryst. Growth. 2001;232:426–431. [Google Scholar]
- (6).Berger BW, Gendron CM, Rlobinson CR, Kaler EW, Lenhoff AM. Acta Crystallogr., Sect. D. 2005;61:724–730. doi: 10.1107/S0907444904029063. [DOI] [PubMed] [Google Scholar]
- (7).Michel H. Trends Biochem. Sci. 1983;8:56–59. [Google Scholar]
- (8).Prive GG. Methods. 2007;41:388–397. doi: 10.1016/j.ymeth.2007.01.007. [DOI] [PubMed] [Google Scholar]
- (9).Song L, Purdy WC. Chem. Rev. 1992;92:1457–1470. [Google Scholar]
- (10).Signorell GA, Kaufmann TC, Kukulski W, Engel A, Remigy HW. J. Struct. Biol. 2007;157:321–328. doi: 10.1016/j.jsb.2006.07.011. [DOI] [PubMed] [Google Scholar]
- (11).DeGrip WJ, VanOostrum J, Bovee-Geurts PHM. Biochem. J. 1998;330:667–674. doi: 10.1042/bj3300667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Li L, Mustafi D, Fu Q, Tereshko V, Chen DLL, Tice JD, Ismagilov RF. Proc. Natl. Acad. Sci. U.S.A. 2006;103:19243–19248. doi: 10.1073/pnas.0607502103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Zheng B, Roach LS, Ismagilov RF. J. Am. Chem. Soc. 2003;125:11170–11171. doi: 10.1021/ja037166v. [DOI] [PubMed] [Google Scholar]
- (14).Duffy DC, McDonald JC, Schueller OJA, Whitesides GM. Anal. Chem. 1998;70:4974–4984. doi: 10.1021/ac980656z. [DOI] [PubMed] [Google Scholar]
- (15).Roach LS, Song H, Ismagilov RF. Anal. Chem. 2005;77:785–796. doi: 10.1021/ac049061w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Otwinowski Z, Minor W. Macromolecular Crystallography, Pt A. Vol. 276. Academic Press; San Diego: 1997. pp. 307–326. [Google Scholar]
- (17).Vagin A, Teplyakov A. J. Appl. Crystallogr. 1997;30:1022–1025. [Google Scholar]
- (18).Collaborative Computational Project, 1994 The CCP4 suite: programs for protein crystallography Acta Crystallogr., Sect. D. 1994;50:760763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- (19).Murshudov GN, Vagin AA, Dodson EJ. Acta Crystallogr., Sect. D. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- (20).Cambillau C, Roussel A. Turbo Frodo, Version OpenGL.1. University Aix-Marseille II; Marseille: 1997. [Google Scholar]
- (21).Emsley P, Cowtan K. Acta Crystallogr., Sect. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- (22).Eriks LR, Mayor JA, Kaplan RS. Anal. Biochem. 2003;323:234–241. doi: 10.1016/j.ab.2003.09.002. [DOI] [PubMed] [Google Scholar]
- (23).Duchene D, Bochot A, Yu SC, Pepin C, Seiller M. Int. J. Pharm. 2003;266:85–90. doi: 10.1016/s0378-5173(03)00384-3. [DOI] [PubMed] [Google Scholar]
- (24).Kantardjieff KA, Rupp B. Protein Sci. 2003;12:1865–1871. doi: 10.1110/ps.0350503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Song H, Chen DL, Ismagilov RF. Angew. Chem., Int. Ed. 2006;45:7336–7356. doi: 10.1002/anie.200601554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Hansen CL, Skordalakes E, Berger JM, Quake SR. Proc. Natl. Acad. Sci. U.S.A. 2002;99:16531–16536. doi: 10.1073/pnas.262485199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Shim JU, Cristobal G, Link DR, Thorsen T, Jia YW, Piattelli K, Fraden S. J. Am. Chem. Soc. 2007;129:8825–8835. doi: 10.1021/ja071820f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


