Abstract
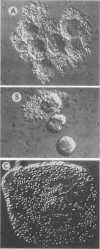
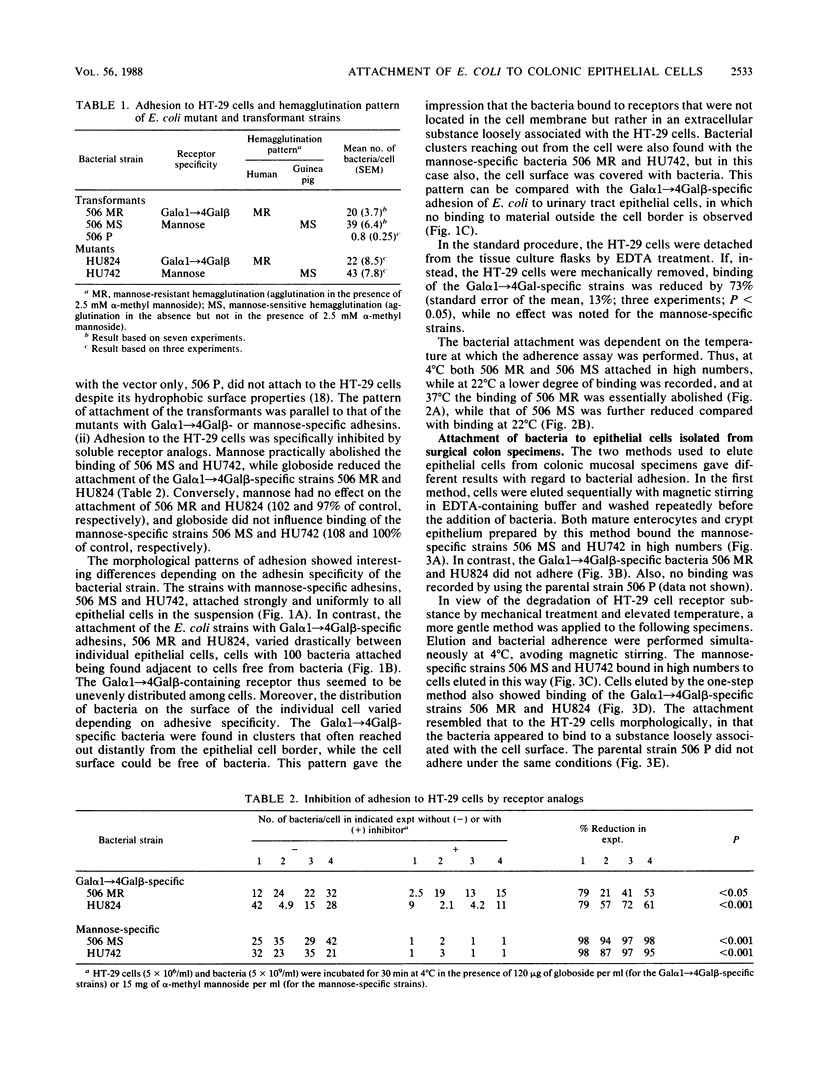
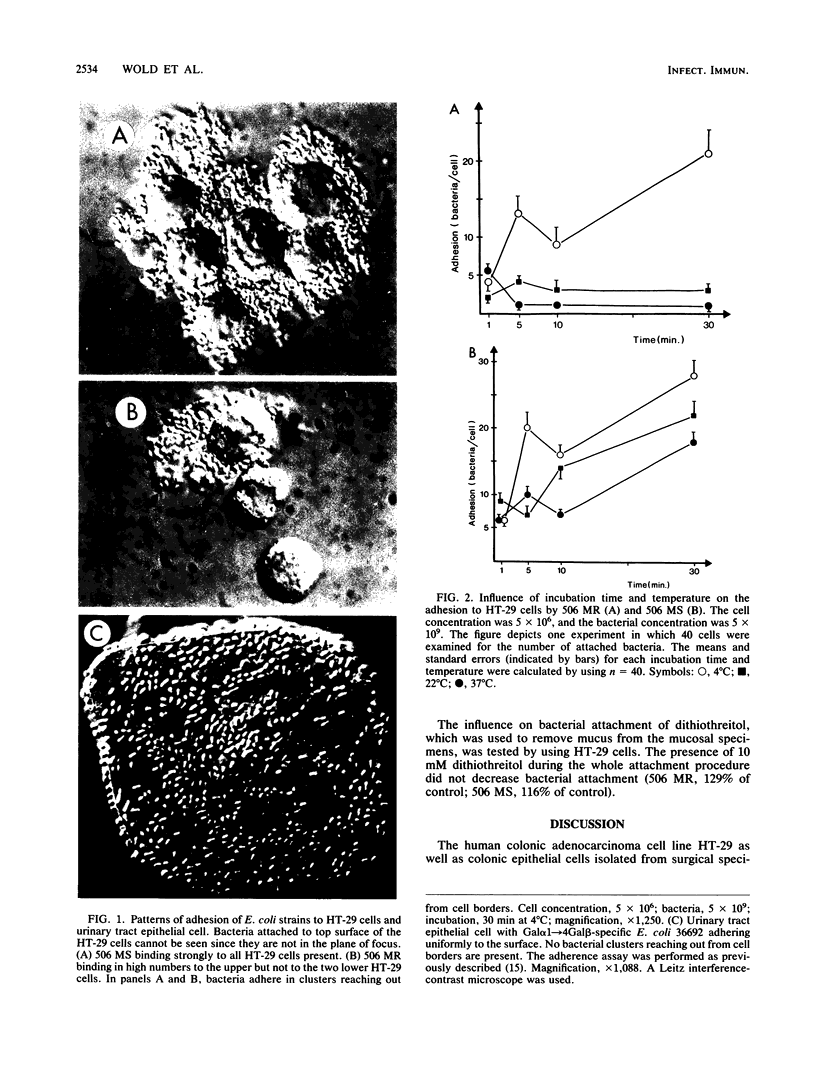
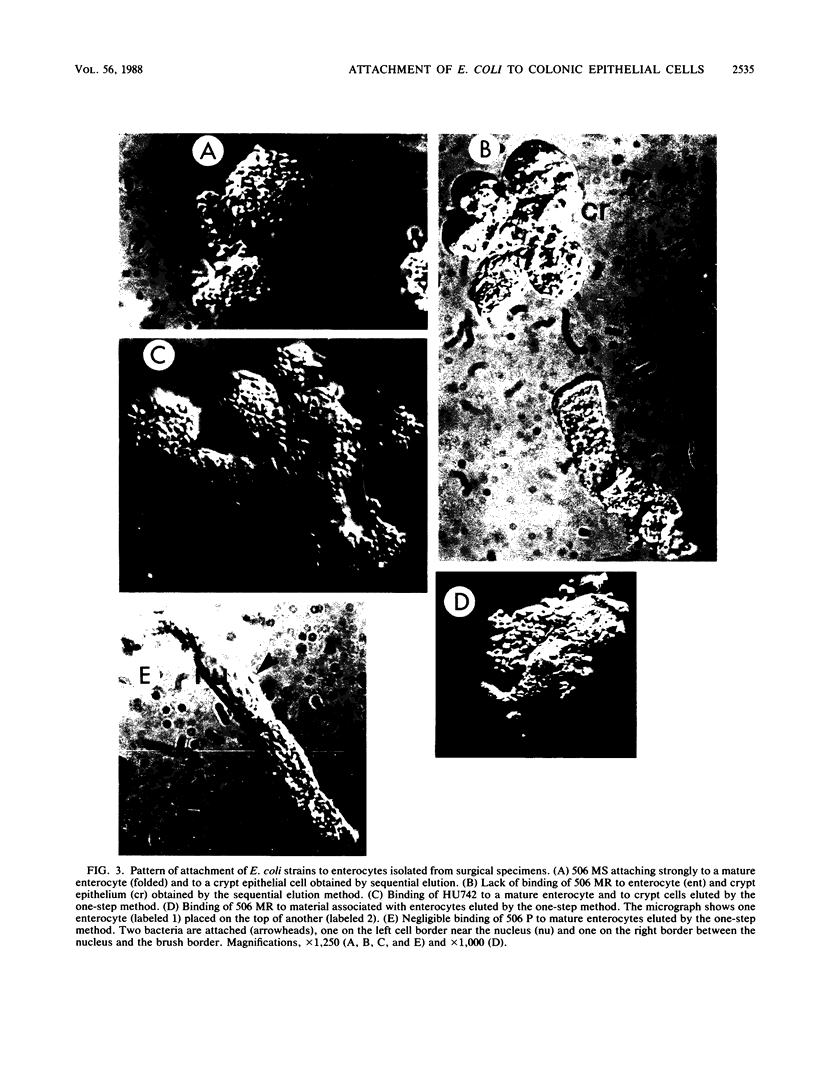
The role of bacterial adhesion for the maintenance of the large-intestinal microflora has not been established. In this study, colonic cells from the adenocarcinoma cell line HT-29 or from surgical specimens were tested for the ability to bind Escherichia coli. The E. coli strains were manipulated by transformation or by mutagenesis to express either mannose-specific type 1 fimbriae (strains 506 MS and HU742) or Gal alpha 1----4Gal beta-specific P fimbriae (506 MR and HU824). Binding to HT-29 cells was seen with strains of either receptor specificity and was inhibited by alpha-methyl mannoside or globotetraosylceramide (GalNAc beta 1----3Gal alpha 1----4Gal beta 1----4Glc-ceramide), respectively. The Gal alpha 1----4Gal beta-specific strains interacted with a loosely surface-associated substance, which was sensitive to mechanical treatment and incubation at 37 degrees C, while the mannose-specific strains bound both directly to the cell and to the loosely associated substance. Isolated colonic epithelial cells bound the mannose-specific bacteria in high numbers, while the attachment of the Gal alpha 1----4Gal beta-specific strains depended on the elution method. Cells eluted sequentially with magnetic stirring were unable to bind the Gal alpha 1----4Gal beta-specific bacteria, while elution by a more gentle method resulted in binding of these strains to material loosely associated with the epithelial cells. Thus, the binding pattern of isolated colonic epithelial cells paralleled that of the HT-29 cell line. Conceivably, binding to mannose- and Gal alpha 1----4Gal beta-containing receptors could contribute to the maintenance of E. coli in the human large intestine.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Eden C. S., Eriksson B., Hanson L. A. Adhesion of Escherichia coli to human uroepithelial cells in vitro. Infect Immun. 1977 Dec;18(3):767–774. doi: 10.1128/iai.18.3.767-774.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edén C. S., Eriksson B., Hanson L. A., Jodal U., Kaijser B., Janson G. L., Lindberg U., Olling S. Adhesion to normal human uroepithelial cells of Escherichia coli from children with various forms of urinary tract infection. J Pediatr. 1978 Sep;93(3):398–403. doi: 10.1016/s0022-3476(78)81145-7. [DOI] [PubMed] [Google Scholar]
- Edén C. S., Freter R., Hagberg L., Hull R., Hull S., Leffler H., Schoolnik G. Inhibition of experimental ascending urinary tract infection by an epithelial cell-surface receptor analogue. Nature. 1982 Aug 5;298(5874):560–562. doi: 10.1038/298560a0. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Jodal U., Korhonen T. K., Lidin-Janson G., Lindberg U., Svanborg Edén C. Adhesion, hemagglutination, and virulence of Escherichia coli causing urinary tract infections. Infect Immun. 1981 Feb;31(2):564–570. doi: 10.1128/iai.31.2.564-570.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley C. L., Neumann C. S., Richmond M. H. Adhesion of commensal bacteria to the large intestine wall in humans. Infect Immun. 1979 Jan;23(1):128–132. doi: 10.1128/iai.23.1.128-132.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S. W., Fogh J., Hong R. Synthesis of secretory component by human colon cancer cells. Scand J Immunol. 1976;5(3):263–268. doi: 10.1111/j.1365-3083.1976.tb00277.x. [DOI] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leffler H., Svanborg-Edén C. Glycolipid receptors for uropathogenic Escherichia coli on human erythrocytes and uroepithelial cells. Infect Immun. 1981 Dec;34(3):920–929. doi: 10.1128/iai.34.3.920-929.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagura H., Nakane P. K., Brown W. R. Translocation of dimeric IgA through neoplastic colon cells in vitro. J Immunol. 1979 Nov;123(5):2359–2368. [PubMed] [Google Scholar]
- Parkkinen J., Rogers G. N., Korhonen T., Dahr W., Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986 Oct;54(1):37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Observations on the pathogenic properties of the K88, Hly and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971 Nov;4(4):467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- Svanborg Eden C., Andersson B., Aniansson G., Leffler H., Lomberg H., Mestecky J., Wold A. E. Glycoconjugate receptors for bacteria attaching to mucosal sites: examples for Escherichia coli and Streptococcus pneumoniae. Adv Exp Med Biol. 1987;216B:931–939. [PubMed] [Google Scholar]
- Svanborg Edén C., Hull R., Falkow S., Leffler H. Target cell specificity of wild-type E. coli and mutants and clones with genetically defined adhesins. Prog Food Nutr Sci. 1983;7(3-4):75–89. [PubMed] [Google Scholar]
- Wadolkowski E. A., Laux D. C., Cohen P. S. Colonization of the streptomycin-treated mouse large intestine by a human fecal Escherichia coli strain: role of adhesion to mucosal receptors. Infect Immun. 1988 May;56(5):1036–1043. doi: 10.1128/iai.56.5.1036-1043.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Wold A. E., Mestecky J., Svanborg Edén C. Agglutination of E. coli by secretory IgA--a result of interaction between bacterial mannose-specific adhesins and immunoglobulin carbohydrate? Monogr Allergy. 1988;24:307–309. [PubMed] [Google Scholar]