The hormone, 17β-estradiol (E2), mediates diverse physiological and regulatory control of reproduction, growth, development, immune response, bone mass, neurological, and cardiovascular health. Carcinomas of the breast, endometrium and ovary are common malignancies in female reproductive tissues, and assessment of their estrogen responsiveness is crucial for prognosis and choice of therapeutic regimen. The classical model of estrogen activation describes ligand binding to receptors ERα/β initiating conformational changes, which result in interactions with transcriptional coregulators and promoter DNA sequences of target genes. The resulting gene transcription provides a physiological response within hours following estrogen exposure. Additional non-genomic estrogen-induced rapid cell signaling pathways have been recognized as important contributors to the overall biological response.1 A new mediator of estrogen-dependent signal transduction, the 7-transmembrane G protein-coupled receptor GPR30, was recently identified.2 This receptor can be co-expressed in cells with ERα/β, or individually in cells lacking classical estrogen receptors. Revankar et. al demonstrated that GPR30 is an intracellular protein, in the endoplasmic reticulum, that binds estrogen with high affinity (Kd ∼6 nM) and mediates rapid cellular responses including calcium mobilization and phosphatidylinositol 3,4,5-trisphosphate production in the nucleus. The role of GPR30 in the growth and proliferation of estrogen responsive tumors is a focus of investigation as a new biomarker and target for cancer diagnostics and therapeutics, and could represent an important imaging target in ERα/β negative cells.3
Non-invasive imaging technology offers great promise for the in vivo characterization of estrogen-dependent tumors. The intracellular location of ERα/β and GPR30 requires neutral, cell permeable imaging agents. Organometallic tricarbonyl-99mTc- and 94Tc-labeled estrogens have potential for diagnostic imaging using single photon emission computed tomography (SPECT) or positron emission tomography (PET).4 Complexes with promising in vitro ERα/β receptor binding affinity have been identified, but poor target tissue uptake and complicated radiolabeling procedures have inhibited further development.4 Lipophilicity and chelate instability are recognized problems, and other significant parameters may be identified through in vitro characterization of estrogen derivatives in cell culture. Herein, we report a new class of tridentate pyridin-2-yl hydrazine Re/99mTc-chelates that exhibit strong binding affinity to GPR30 and ERα/β, and evaluate intracellular binding with a functional cell-based receptor-mediated signaling assay.
Alkylation of the di-tboc 5-bromopyridin-2-yl hydrazine4c with tbutyl-bromoacetate followed by deprotection with trifluoroacetic acid gave the desired chelate in 90% yield. This ligand formed the neutral tridentate complex 1 in 87% yield from tricarbonyl-Re(I) in aqueous ethanol.5 Single crystals of 1 suitable for X-ray crystallography were grown by slow evaporation from ethyl acetate. The structure shown in Figure 1 exhibits an undistorted octahedral coordination geometry with tridentate facial orientation of pyridyl, hydrazine-N' and carboxylate groups.
Figure 1.
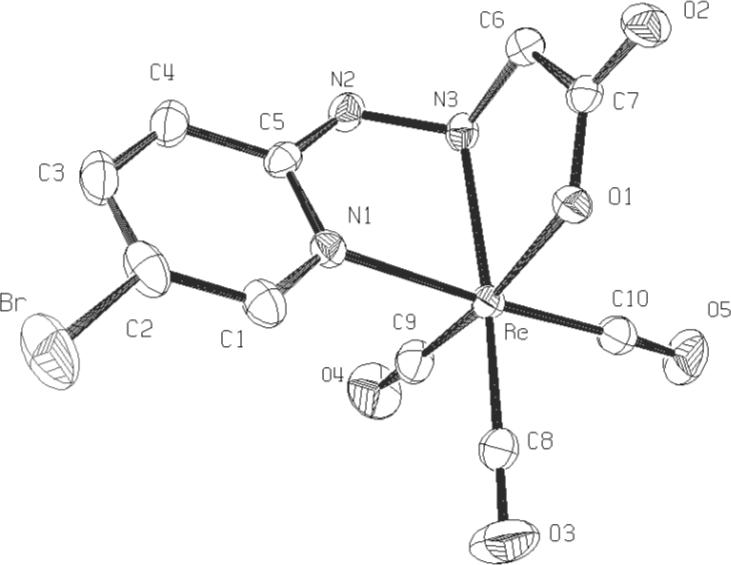
X-ray ORTEP rendition of complex 1
Sonogashira coupling of the protected chelate with 17α-ethynylestradiol, followed by deprotection, and Re(CO)3+ labeling gave 2 in 95% yield. The complex was characterized by HPLC-MS ESI-negative detection as two chelate diastereomers (m/z 730, 9.55 and 10.50 min) as expected from the facial coordination geometry, but were not isolated separately. Stereospecific hydrogenation of 2 using Lindlar's catalyst produced complex 3 containing a (Z)-ethene linkage in 70% yield. Hydrogenation of 2 with Pd/C gave the saturated alkane-linked complex 4 in 98% yield.
The receptor binding affinities of 2−4 for full length human ERα and ERβ were determined by competitive radiometric assays with [3H]estradiol and expressed as relative binding affinity (RBA) compared with 17β-estradiol (100%). The connecting linkage affected the binding affinity and selectivity of the complexes for ERα/β. The linear alkyne complex 2 exhibited high affinity and four-fold greater selectivity for ERα over ERβ. The Z-alkene complex 3 had greater affinity and two-fold selectivity for ERβ. These RBA values compare favorably with the best examples of reported estradiol tricarbonyl-Re(I) complexes.4 Complex 4 had low affinity for both ERα/β that can be attributed to unfavorable steric and entropic effects of the flexible alkyl linkage with receptor binding. These results indicate that relatively minor changes in the linkage can affect binding affinity and selectivity, and could be optimized to increase ER subtype selectivity as demonstrated for selective estrogen receptor modulators.6
The GPR30 binding affinity of 2 and 3 was determined using a fluorescent Alexa633-labeled conjugate of estradiol that binds to GPR30 and ERα/β.2c Binding affinity was assessed by flow cytometry using GPR30-transfected COS7 cells that do not express endogenous GPR30 or ERα/β. The cells were permeabilized with saponin to enable access of the charged fluorescent estrogen. Competitive binding was evaluated using 17β-estradiol as a positive control for specific binding of the fluorescent estrogen to GPR30 Fig 2a, and the relative Ki values of 2 and 3 are reported in Table 1. The non-binding diastereomer 17α-estradiol was included as a negative control. Both complexes 2 and 3 exhibited strong binding to GPR30 and demonstrate the potential for targeting this receptor.
Fig 2.
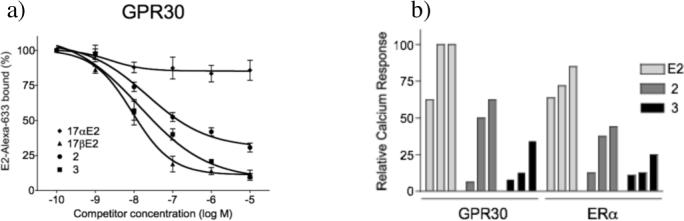
Fluorescence assays for GPR30 binding and activation with 2 and 3. a) GPR30 competitive binding of E2, 17α-E2, 2, and 3 with fluorescent estradiol derivative E2Alexa using permeabilized COS7 cells. b) Calcium mobilization assay for GPR30 and ERα in transfected COS7 cells vs concentration of E2, and complexes 2 and 3 (each at 10, 100, and 1000 nM).
Table 1.
Structure and relative binding affinity of estradiol complexes 2, 3, 4 for ERα/β and GPR30
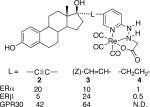 |
To assess receptor binding in whole cells we employed a functional assay based on the rapid receptor-mediated mobilization of intracellular calcium elicited by estrogen ligand binding to ERα and GPR30. Transfected COS7 cells expressing ERα or GPR30 were incubated at room temperature with the calcium-responsive fluorescent dye indo1-AM. Dose-dependent fluorescence was measured at 10, 100, and 1000 nM extracellular concentrations of E2, 2 and 3 (Figure 2b). Alkyne complex 2 initiated a rapid increase in intracellular calcium concentrations with both ERα and GPR30. The Z-alkene complex 3 produced lower calcium levels during the same time period. These results demonstrate that complexes 2 and 3 are able to penetrate the cell membrane, bind and activate the targeted estrogen receptors. Cell permeability, a critical parameter affecting the kinetics of tissue uptake and overall biodistribution, can be assessed using this type of functional assay.
Convenient methodology for labeling with [99mTc(CO)3(H2O)3)]+ has been developed, and the IsoLink® kit from Mallinckrodt reliably produced the aquo complex with radiochemical purity ≥ 98% (n ≥ 20).7 Due to the acid sensitivity of the tertiary propargylic 17-β-alcohol of the estradiol chelate, the alkaline mixture was neutralized with acetic acid rather than HCl. 99mTc-labeling was conducted at ambient temperature for 2 hr. Solid phase extraction using a C-18 Sep-Pak® with 40% EtOH/H2O effectively removed excess ligand, and the 99mTc-complex 2 eluted with EtOH in radiochemical yields ranging from 90−95% of the total loaded radioactivity. The radiochemical purity assessed by HPLC was ≥ 85% (n ≥ 10), and chromatographic resolution of the two diastereoisomeric chelates was observed by radiometric detection. The specific activity determined post-purification was 40 mCi/μg. HPLC and ITLC analyses demonstrated no significant degradation, reoxidation or “leak” of 99mTc from the chelate after 48 hours of storage at 4 °C, 25 °C and 37°C. The complex exhibited a log P octanol/water coefficient of 3.87 ± 0.49 (n = 4) determined by shake flask method, that corresponds closely with estradiol log Po/w = 4.01. The stability of 99mTc-2 was also evaluated in the presence of an 100-fold excess of histidine (1 mM). The radiochemical purity accessed via HPLC after 24 hr incubation at 37° C was 76.32% ± 2.81 (n = 3) with less than 1% reoxidation of the 99mTc species.
These results demonstrate that chelates 2 and 3 interact with receptors GPR30 and ERα/β in whole cells, and suggests increased use of in vitro assays may facilitate the development of targeted imaging agents for intracellular receptors.
Supplementary Material
ACKNOWLEDGMENT
NIH/SCORE GM08136 (J.B.A.), CA116662 and UNM CRTC Translational Research Award (E. R. P.), CA25836 (J. A. K.), Cowboys for Cancer Research Foundation. WM Keck Foundation and NIH Roadmap U54MH074425.
Footnotes
Supporting Information Available: Experimental and spectral data provided as pdf files, X-ray structure of 2 provided as a cif file.
References
- 1.a Losel RM, Falkenstein E, Feuring M, Schultz A, Tillmann H-C, Rossol-Haseroth K, Wehling M. Physiol. Rev. 2003;83:965–1016. doi: 10.1152/physrev.00003.2003. [DOI] [PubMed] [Google Scholar]; b Osborne CK, Schiff R. J. Clin. Oncol. 2005;23:1616–1622. doi: 10.1200/JCO.2005.10.036. [DOI] [PubMed] [Google Scholar]; c Ariazi EA, Ariazi JL, Cordera F, Jordan VC. Curr. Top. Med. Chem. 2006;6:181–202. [PubMed] [Google Scholar]
- 2.a Filardo EJ, Quinn JA, Bland KI, Frackelton AR. Mol. Endocrin. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]; b Thomas P, Pang Y, Filardo EJ, Dong J. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]; c Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 3.Prossnitz ER, Arterburn JB, Edwards BS, Sklar LA, Oprea TI. Expert Opin. Drug Discov. 2006;1:137–150. doi: 10.1517/17460441.1.2.137. [DOI] [PubMed] [Google Scholar]
- 4.a Top S, El Hafa H, Vessieres A, Quivy J, Vaissermann J, Hughes DW, Mcglinchey MJ, Mornon JP, Thoreau E, Jaouen G. J. Am. Chem. Soc. 1995;117:8372–8380. [Google Scholar]; b Luyt LG, Bigott HM, Welch MJ, Katzenellenbogen JA. Biorg. Med. Chem. Lett. 2003;11:4977–4989. doi: 10.1016/j.bmc.2003.09.004. [DOI] [PubMed] [Google Scholar]; c Arterburn JB, Corona C, Rao KV. J. Org. Chem. 2003;68:7063–7070. doi: 10.1021/jo034780g. [DOI] [PubMed] [Google Scholar]; d Top S, Boubekeur L, Jaouen G, Mundwiler S, Spingler B, Alberto R. Eur. J. Inorg. Chem. 2004:2013–2017. [Google Scholar]; e Bigott HM, Parent E, Luyt LG, Katzenellenbogen JA, Welch MJ. Bioconjugate Chem. 2005;16:255–264. doi: 10.1021/bc049770g. [DOI] [PubMed] [Google Scholar]
- 5.Lazarova N, James S, Babich J, Zubieta J. Inorg. Chem. Commun. 2004;7:1023–1026. [Google Scholar]
- 6.Manas ES, et al. J. Am. Chem. Soc. 2004;126:15106–15119. doi: 10.1021/ja047633o. [DOI] [PubMed] [Google Scholar]
- 7.a Alberto R, Schibli R, Egli A, Schubiger AP. J. Am. Chem. Soc. 1998;120:7987–7988. [Google Scholar]; b Schibli R, Schubiger PA. Eur J Nucl Med Mol Imaging. 2002;29:1529–1542. doi: 10.1007/s00259-002-0900-8. [DOI] [PubMed] [Google Scholar]; c Stichelberger A, Waibel R, Dumas C, Schubiger PA, Schibli R. Nucl. Med. Biol. 2005;30:465–470. doi: 10.1016/s0969-8051(03)00032-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


