Abstract
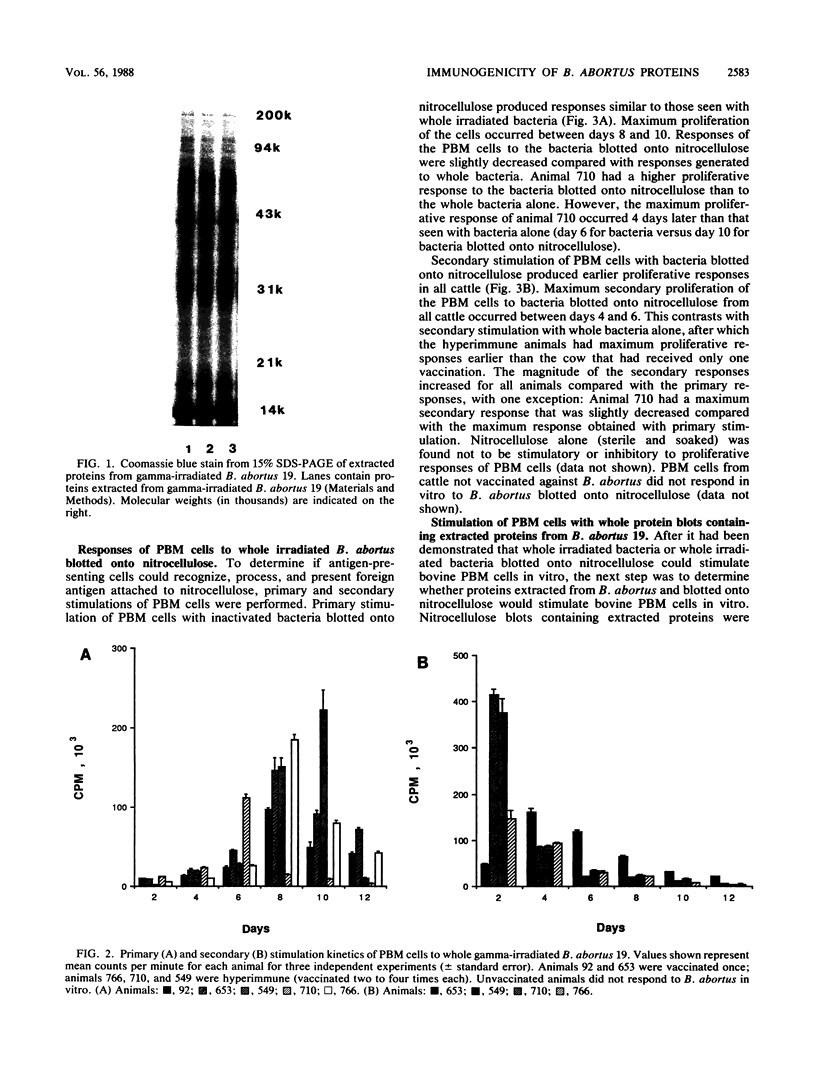
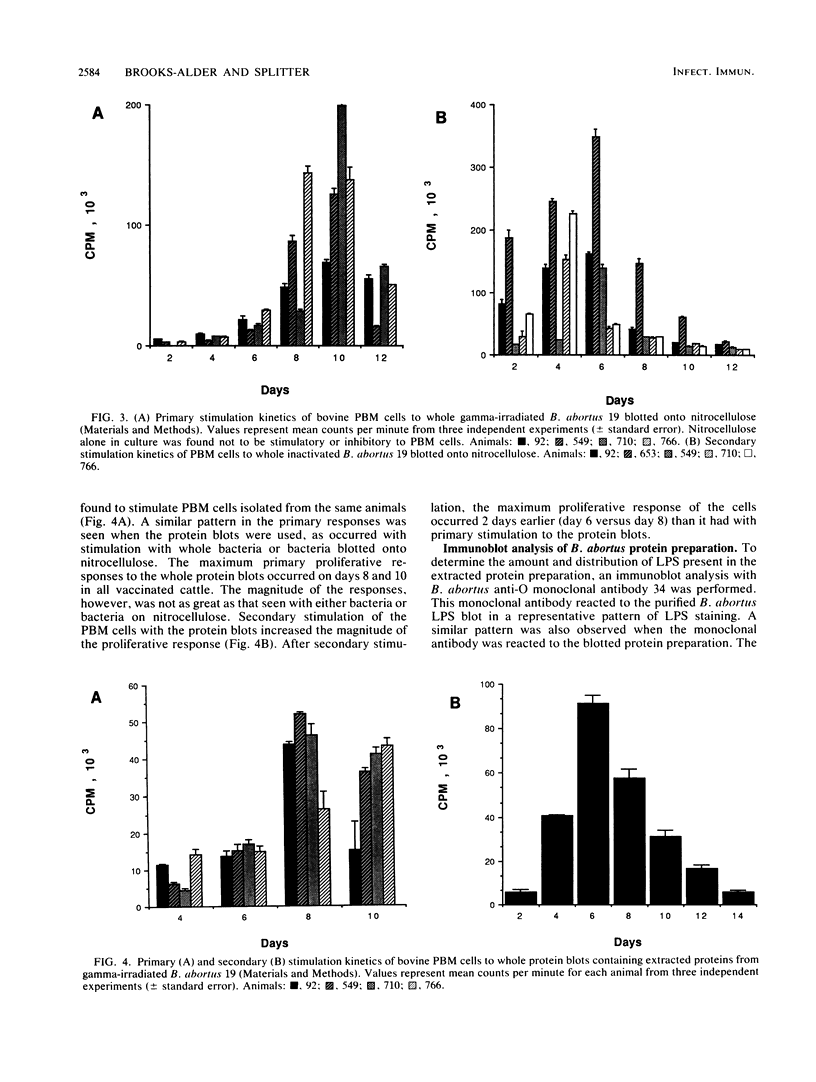
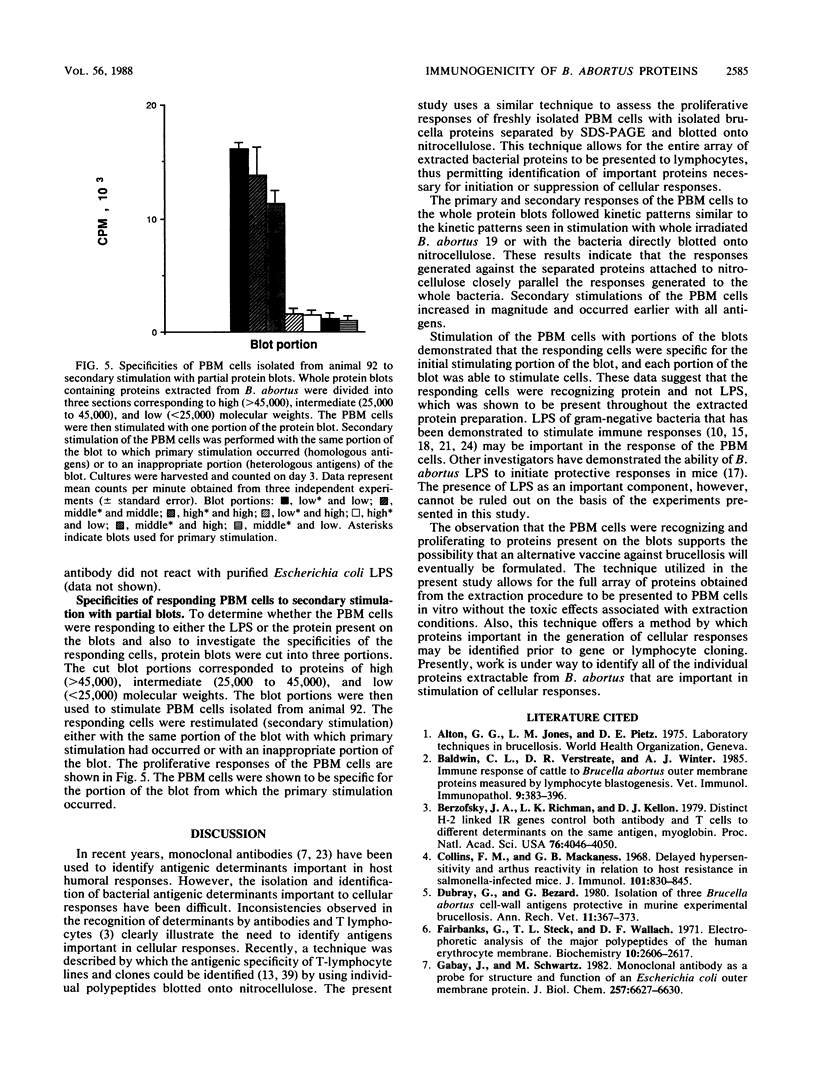
Isolation and identification of Brucella antigenic determinants important to cellular responses have been difficult. In this study, bovine peripheral blood mononuclear (PBM) cells from cattle vaccinated with Brucella abortus 19 proliferated to extracted bacterial proteins blotted onto nitrocellulose. Proteins were extracted from gamma-irradiated B. abortus 19 with a sodium dodecyl sulfate extraction buffer. The extracted proteins were separated electrophoretically by sodium dodecyl sulfate-polyacrylamide gel electrophoresis prior to electroblotting onto nitrocellulose. Nitrocellulose sections corresponding to individual lanes of the gel (containing all separated proteins) were then cultured with the PBM cells. Primary and secondary stimulation responses of the PBM cells with the whole protein blots were similar kinetically to the responses of the PBM cells stimulated with whole irradiated B. abortus 19 or with whole irradiated B. abortus 19 blotted onto nitrocellulose. Although lipopolysaccharide was determined to be associated with the extracted proteins and transferred onto the blots, the lipopolysaccharide did not stimulate cellular proliferation, as indicated by the antigen-specific secondary responses. Stimulating PBM cells with portions of the blot containing high (greater than 45,000)-, medium (25,000 to 45,000)- or low (25,000)-molecular-weight proteins demonstrated that the responding cells were specific only to the proteins of corresponding molecular weights. These results indicate that cellular responses to individual proteins can be studied without cloning the bacterial genes or purifying the individual proteins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baldwin C. L., Verstreate D. R., Winter A. J. Immune response of cattle to Brucella abortus outer membrane proteins measured by lymphocyte blastogenesis. Vet Immunol Immunopathol. 1985 Aug;9(4):383–396. doi: 10.1016/0165-2427(85)90067-4. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Richman L. K., Killion D. J. Distinct H-2-linked Ir genes control both antibody and T cell responses to different determinants on the same antigen, myoglobin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4046–4050. doi: 10.1073/pnas.76.8.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. Delayed hypersensitivity and arthus reactivity in relation to host resistance in salmonella-infected mice. J Immunol. 1968 Nov;101(5):830–845. [PubMed] [Google Scholar]
- Dubray G., Bézard G. Isolation of three Brucella abortus cell-wall antigens protective in murine experimental brucellosis. Ann Rech Vet. 1980;11(4):367–373. [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- Gabay J., Schwartz M. Monoclonal antibody as a probe for structure and function of an Escherichia coli outer membrane protein. J Biol Chem. 1982 Jun 25;257(12):6627–6630. [PubMed] [Google Scholar]
- Gamazo C., Moriyón I. Release of outer membrane fragments by exponentially growing Brucella melitensis cells. Infect Immun. 1987 Mar;55(3):609–615. doi: 10.1128/iai.55.3.609-615.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jian H., Chu T. P. A study of immunological cross-reaction between Yersinia enterocolitica 0:9 and Brucella abortus. Dev Biol Stand. 1984;56:179–183. [PubMed] [Google Scholar]
- Kuusi N., Nurminen M., Saxén H., Mäkelä P. H. Immunization with major outer membrane protein (porin) preparations in experimental murine salmonellosis: effect of lipopolysaccharide. Infect Immun. 1981 Nov;34(2):328–332. doi: 10.1128/iai.34.2.328-332.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Young D. B. A novel approach to the identification of T-cell epitopes in Mycobacterium tuberculosis using human T-lymphocyte clones. Immunology. 1987 Jan;60(1):1–5. [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning P. A., Bartowsky E. J., Leavesly D. I., Hackett J. A., Heuzenroeder M. W. Molecular cloning using immune sera of a 22-kDal minor outer membrane protein of Vibrio cholerae. Gene. 1985;34(1):95–103. doi: 10.1016/0378-1119(85)90299-9. [DOI] [PubMed] [Google Scholar]
- Montaraz J. A., Winter A. J., Hunter D. M., Sowa B. A., Wu A. M., Adams L. G. Protection against Brucella abortus in mice with O-polysaccharide-specific monoclonal antibodies. Infect Immun. 1986 Mar;51(3):961–963. doi: 10.1128/iai.51.3.961-963.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moryión I., Berman D. T. Interaction of Escherichia coli matrix protein with Brucella abortus peptidoglycan and chitin. Dev Biol Stand. 1984;56:227–234. [PubMed] [Google Scholar]
- Nano F. E., Barstad P. A., Mayer L. W., Coligan J. E., Caldwell H. D. Partial amino acid sequence and molecular cloning of the encoding gene for the major outer membrane protein of Chlamydia trachomatis. Infect Immun. 1985 May;48(2):372–377. doi: 10.1128/iai.48.2.372-377.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov H., Hogarth M., McKenzie I. F., Cheers C. In vivo and in vitro effects of monoclonal antibody to Ly antigens on immunity to infection. Cell Immunol. 1982 Jul 15;71(1):127–138. doi: 10.1016/0008-8749(82)90502-0. [DOI] [PubMed] [Google Scholar]
- Peavy D. L., Shands J. W., Jr, Adler W. H., Smith R. T. Mitogenicity of bacterial endotoxins: characterization of the mitogenic principle. J Immunol. 1973 Aug;111(2):352–357. [PubMed] [Google Scholar]
- Person J. M., Frottier J., le Garrec Y., Barrat F., Bastin R., Pilet C. Exploration of the cellular mediated immunity by the blastogenesis test during chronic brucellosis in human. Comp Immunol Microbiol Infect Dis. 1987;10(1):1–8. doi: 10.1016/0147-9571(87)90035-x. [DOI] [PubMed] [Google Scholar]
- Robertson S. M., Frisch C. F., Gulig P. A., Kettman J. R., Johnston K. H., Hansen E. J. Monoclonal antibodies directed against a cell surface-exposed outer membrane protein of Haemophilus influenzae type b. Infect Immun. 1982 Apr;36(1):80–88. doi: 10.1128/iai.36.1.80-88.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertsson J. A., Fossum C., Svenson S. B., Lindberg A. A. Salmonella typhimurium infection in calves: specific immune reactivity against O-antigenic polysaccharide detectable in in vitro assays. Infect Immun. 1982 Aug;37(2):728–736. doi: 10.1128/iai.37.2.728-736.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson G., Leavesley D. I., Lagnado C. A., Heuzenroeder M. W., Manning P. A. Purification of the 25-kDa Vibrio cholerae major outer-membrane protein and the molecular cloning of its gene: ompV. Eur J Biochem. 1985 Apr 15;148(2):385–390. doi: 10.1111/j.1432-1033.1985.tb08850.x. [DOI] [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L. Biochemical and biological properties of soluble protein preparations from Brucella abortus. Dev Biol Stand. 1984;56:199–211. [PubMed] [Google Scholar]
- Tabatabai L. B., Deyoe B. L. Characterization of salt-extractable protein antigens from Brucella abortus by crossed immunoelectrophoresis and isoelectricfocusing. Vet Microbiol. 1984 Oct;9(6):549–560. doi: 10.1016/0378-1135(84)90017-8. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Frasch C. E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982 Jan 1;119(1):115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- Udhayakumar V., Muthukkaruppan V. R. An outer membrane protein (porin) as an eliciting antigen for delayed-type hypersensitivity in murine salmonellosis. Infect Immun. 1987 Mar;55(3):822–824. doi: 10.1128/iai.55.3.822-824.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udhayakumar V., Muthukkaruppan V. R. Protective immunity induced by outer membrane proteins of Salmonella typhimurium in mice. Infect Immun. 1987 Mar;55(3):816–821. doi: 10.1128/iai.55.3.816-821.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Creasy M. T., Caveney N. T., Baldwin C. L., Blab M. W., Winter A. J. Outer membrane proteins of Brucella abortus: isolation and characterization. Infect Immun. 1982 Mar;35(3):979–989. doi: 10.1128/iai.35.3.979-989.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verstreate D. R., Winter A. J. Comparison of sodium dodecyl sulfate-polyacrylamide gel electrophoresis profiles and antigenic relatedness among outer membrane proteins of 49 Brucella abortus strains. Infect Immun. 1984 Oct;46(1):182–187. doi: 10.1128/iai.46.1.182-187.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter A. J., Verstreate D. R., Hall C. E., Jacobson R. H., Castleman W. L., Meredith M. P., McLaughlin C. A. Immune response to porin in cattle immunized with whole cell, outer membrane, and outer membrane protein antigens of Brucella abortus combined with trehalose dimycolate and muramyl dipeptide adjuvants. Infect Immun. 1983 Dec;42(3):1159–1167. doi: 10.1128/iai.42.3.1159-1167.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff W. A., Parr T. R., Jr, Hancock R. E., Hanne L. F., Nicas T. I., Iglewski B. H. Expression in Escherichia coli and function of Pseudomonas aeruginosa outer membrane porin protein F. J Bacteriol. 1986 Aug;167(2):473–479. doi: 10.1128/jb.167.2.473-479.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. B., Lamb J. R. T lymphocytes respond to solid-phase antigen: a novel approach to the molecular analysis of cellular immunity. Immunology. 1986 Oct;59(2):167–171. [PMC free article] [PubMed] [Google Scholar]



