Abstract
Mimetic species evolve colours and body patterns to closely resemble poisonous species and thus avoid predation (Batesian mimicry), or resemble beneficial or harmless species in order to approach and attack prey (aggressive mimicry). Facultative mimicry, the ability to switch between mimic and non-mimic colours at will, is uncommon in the animal kingdom, but has been shown in a cephalopod, and recently in a marine fish, the bluestriped fangblenny Plagiotremus rhinorhynchos, an aggressive mimic of the juvenile cleaner fish Labroides dimidiatus. Here we demonstrate for the first time that fangblennies adopted mimic colours in the presence of juvenile cleaner fish; however, this only occurred in smaller individuals. Field data indicated that when juvenile cleaner fish were abundant, the proportion of mimic to non-mimic fangblennies was greater, suggesting that fangblennies adopt their mimic disguise depending on the availability of cleaner fish. Finally, measurements of spectral reflectance suggest that not only do mimic fangblennies accurately resemble the colour of their cleaner fish models but also mimic other species of fish that they associate with. This study provides insights into the cues that control this remarkable facultative mimicry system and qualitatively measures its accuracy.
Keywords: aggressive mimicry, coral reef fish, signal accuracy, cleaner wrasse, dynamic mimicry
1. Introduction
Animals use colour signals for a wide range of functions including signalling willingness to mate, territoriality, sexual selection, crypsis and advertising unpalatability to potential predators (e.g. Cott 1940; Endler 1978; Bennett et al. 1997). Colour patterns may be permanent throughout the lifetime of an animal, may change with different ontogenetic stages or may be physiologically adapted to changes in the environment or to the ‘mood’ of the individual (e.g. Hanlon & Messenger 1988; Armbruster & Page 1996). However, mimetic species, defined as those that closely resemble another species in order to avoid predation (protective mimicry), or to attack prey (aggressive mimicry), usually exhibit permanent colour patterns or are only mimetic for part of their lifetime. In contrast, facultative (or dynamic) mimicry, the remarkable ability to switch on mimic coloration at will or change appearance to mimic a variety of species, has only recently been described in the following two species.
In the invertebrate kingdom, the ‘mimic octopus’ can resemble a variety of poisonous species including banded sea snake (Laticauda sp.) and lionfish (Pterosis sp.; Norman et al. 2001). In addition, individuals also use a general background camouflage when predators pass close by. The only reported example of facultative mimicry of another species in a vertebrate is from a marine fish, the bluestriped fangblenny Plagiotremus rhinorhynchos (Côté & Cheney 2005), which is an aggressive mimic of the juvenile cleaner fish Labroides dimidiatus (Côté & Cheney 2004; Moland & Jones 2004). Instead of cleaning reef fish by removing ectoparasites and mucus from larger reef fish clients (Côté 2000), fangblennies attack fish removing scales and dermal tissue (Kuwamara 1981). In its mimic colour form, the bluestriped fangblenny exhibits a black body with one electric blue lateral stripe, closely resembling the juvenile cleaner fish. In its non-mimic colour form, fangblennies are often found associated with shoals of differently coloured fishes (Côté & Cheney 2005). From a human's perspective, they appear to closely match the colours of the fishes they associate with (Côté & Cheney 2005), which may protect them from detection by potential victims or predators. However, using non-subjective colour measurement is crucial, as there is ample evidence that a human interpretation of animal visual signals is not adequate (Lythgoe 1979; Endler 1990). Although non-subjective measurements have been conducted in the marine environment to provide valuable insights to visual signalling (e.g. Losey 2003; Siebeck 2004), to our knowledge, such assessments have not been carried out to examine colours in marine mimicry systems.
Little is known about what factors influence the facultative changes in coloration of bluestriped fangblennies. Recent studies have found that bluestriped fangblennies lose their mimetic coloration when a cleaner fish is removed from a cleaning station (Moland & Jones 2004) or when the fangblenny is translocated away from a cleaner fish (Côté & Cheney 2005). However, colour change has only been shown in one direction (from mimic to non-mimic) and it is unknown whether the presence of the model directly functions as a visual cue for a fangblenny to switch from a non-mimic to a mimic colour. Furthermore, fangblennies possibly have a wide range of disguises that can be used depending on the availability of fishes or type of habitat (Côté & Cheney 2005). Therefore, by understanding colour change and accuracy of signals between fangblennies and other fishes that they associate with will help us to understand this remarkable example of mimicry in the vertebrate kingdom. If fangblennies do alter to a mimic colour in the presence of juvenile cleaner fish, we predict that in the field the ratio of mimics to non-mimics should increase as the model abundance increases, if the model is present in low densities.
Hence, in this study we examined: (i) whether cleaner fish act as a cue for colour change in the bluestriped fangblenny, (ii) whether the proportion of mimic to non-mimic fangblennies was dependent on the number of juvenile cleaner fish in the field, and (iii) using non-subjective colour analyses, whether the mimic form of bluestriped fangblenny was an accurate mimic of the juvenile cleaner fish, and whether non-mimic fangblennies closely matched the body colours of other fishes they associated with.
2. Material and methods
This study was conducted at the Lizard Island Research Station and on reefs around Lizard Island, Great Barrier Reef, Australia during April to May 2004, July 2005 and December to January 2005 to 2006. Fishes were collected from shallow reefs (2–10 m) with hand and barrier nets.
(a) Colour changes in aquarium trials
Thirty-four fangblennies were randomly located and captured with a hand net and their standard length (SL) was measured. Original body colours were subjectively (as interpreted by the human eye) recorded at capture, and then any subsequent changes in body colour were recorded at 5, 10, 15, 30 and 60 min, then approximately every 2 h after capture. Each fangblenny was categorized as a mimic colour form (black body with one neon blue lateral stripe) or a non-mimic colour form (consisting of a brown, olive or orange body with two white or light blue/green lateral stripes; Côté & Cheney 2005). We also recorded the number and species of fishes that were located in a 2×2 m quadrat surrounding the fangblenny at capture. Fangblennies were then held in aquaria with running seawater and left to acclimatize for 2–3 days. In turn, fangblennies were placed in aquaria (0.7×0.5×0.3 m) for 1–2 days with a juvenile cleaner, a control wrasse (juvenile Halichoeres melanurus), 10 lemon damselfish (Pomacentrus moluccensis) or no fishes, to determine whether other fishes would act as cues for fangblennies to change colour. Ten damselfish were used in contrast to the single fish used in the other treatments in an attempt to replicate a shoal of P. moluccensis, the usual way in which these common fish occur (Allen et al. 2003). The treatment order and treatment in each particular tank was randomized. The colour of the fangblenny was noted frequently: every 15 min for the first 3 h and then every 1–2 h during daylight hours.
(b) Relative estimates of fishes in the field
To assess whether the ratio of mimic to non-mimic fangblennies was dependent on the number of juvenile cleaner fish in the surrounding environment, fish counts were conducted on 10 patch reefs around Lizard Island. Three 20-min swims in one general direction at each patch reef were conducted at a constant speed by the same observer. The relative numbers of mimic (black/blue), non-mimic fangblennies (other colour forms) and juvenile cleaner fish were counted. The size of each fangblenny and juvenile cleaner fish was estimated to the nearest 5 mm. In a preliminary study, fish length was estimated by the observer and compared with actual length measured by a ruler (n=25). Percentage differences in the estimated and actual length were calculated (mean±s.e.: 6.2±2.4%), and was deemed accurate enough for this study. Data were then averaged for each patch reef. Counts were conducted during April 2004, July 2005 and January 2006.
(c) Spectrophotometry of fish colours
The colours of bluestriped fangblennies, juvenile cleaner fish and other associated fishes (also caught with hand and barrier nets) were measured with an Ocean Optics USB2000 spectrometer (Ocean Optics, Dunedin, FL), while in aquaria, fishes were caught in a hand net and held in the water. Many fishes alter their coloration under stressful conditions; however, all fishes measured in this study rarely changed colour within 5 min of capture. Therefore, spectral reflectance measurements were conducted immediately after capture to ensure that potential colour changes due to stress were minimized. The light reflected from each differently coloured area of the fishes was then measured through a bifurcated fibre optic cable connected to a PX-2 pulsed xenon light source and stored by the spectrometer, which was connected to a laptop computer running OOIBase32 software (Ocean Optics, Dunedin, FL). The bare end of the fibre was placed close to the fish so that it was sampled from that colour region alone and at a 45° angle to avoid specular reflection. Colour measurements were taken within each coloured body patch greater than 4 mm2; therefore, finely barred, streaked or mottled patterns were ignored. Each measurement was averaged from at least 10 samples of each coloured area of the fish. Spectra from at least five individuals were averaged. Two orange fangblennies that were collected had altered their body colours by the time they were transported to the aquaria and therefore their colours could not be measured. For further methods on spectrophotometry, see Endler (1990) and Marshall et al. (2003).
(d) Statistical analyses
We used a binomial generalized linear model with complimentary log–log link (McCullagh & Nelder 1989) to determine whether the proportion of mimic fangblennies was dependent on the number of juvenile cleaner fish. The proportion of mimic fangblennies was used as the dependent variable; month (April, July or January) as a fixed factor to account for seasonal variation; number of juvenile cleaner fish, total number of fangblennies and average size of fangblennies on each random swim were covariates. Non-significant terms were eliminated by stepwise deletion to obtain a minimal adequate model. Normal quantile–quantile plots of the residuals and plots of the residuals versus the fitted values were examined to check the assumptions of normality and heteroskedasticity, respectively. All statistical analyses were conducted with R (R Foundation for Statistical Computing, Vienna; http://www.R-project.org).
3. Results
(a) Colour changes in aquarium trials
We collected 34 fangblennies that ranged in size from 35 to 80 mm (mean±s.d.: 49.9±12.0). On capture, there was no significant difference in the size of fangblennies in relation to their colours (ANOVA F3,31=2.34, p=0.10; table 1). After capture from the field, 22 out of 34 individuals changed colour within 5–60 min (table 1). None of the fish caught maintained their initial black/blue coloration or orange colour for more than 60 min. Fish that initially changed colour were significantly smaller than those that did not change colour (mean±s.d. (mm); colour change: 45.6±7.3; no colour change: 59.2±14.0; independent t-test: t14.2=3.16, p=0.007).
Table 1.
Changes in body colour of P. rhinorhynchos after capture.
| colour after 60 min | ||||||
|---|---|---|---|---|---|---|
| standard length (SL) mean±s.d. (mm) | black | brown | olive | orange | ||
| body colour when caught | black (9) | 44.2±3.1 | 0 | 7 | 2 | 0 |
| brown (7) | 53.6±12.6 | 0 | 6 | 1 | 0 | |
| olive (16) | 53.5±13.3 | 0 | 10 | 6 | 0 | |
| orange (2) | 38.0±2.8 | 0 | 0 | 2 | 0 | |
In aquarium trials, no fish changed colour after capture with a hand net within 5 min, which could have occurred due to stress and has been shown to occur in other non-mimetic fish (K. L. Cheney & N. J. Marshall 2006, personal observations). When placed in a tank with a juvenile cleaner fish, 10 out of 34 non-mimic fangblennies changed to a mimic black and blue colour. This occurred within 5–30 min (mean±s.d.: 10.0±7.8 min). When originally caught, these individuals were of varying colours (five mimic black/blue and five non-mimic fangblennies: two brown and three olive) and were smaller individuals less than 50 mm (mean±s.d. (mm): changed to mimic colour: 42.5±4.0, n=10; no change to mimic colour: 53.1±12.6, n=25; t32.19=3.84, p=0.001). In nine out of the 10 cases where fangblennies changed to the mimic coloration, the black and blue coloration was maintained until the fangblenny or the juvenile cleaner fish was removed from the tank. Fangblennies then returned to their original ‘pre-contact with juvenile cleaner fish’ colour (brown or olive) within 30 min. In one case, the fangblenny lost its black and blue coloration and became brown and white after being in the tank with the juvenile cleaner fish for 4 h. No colour changes were observed in any fangblenny (n=34) when placed with a single H. melanurus, P. moluccensis or no fish.
(b) Relative estimates of fishes in the field
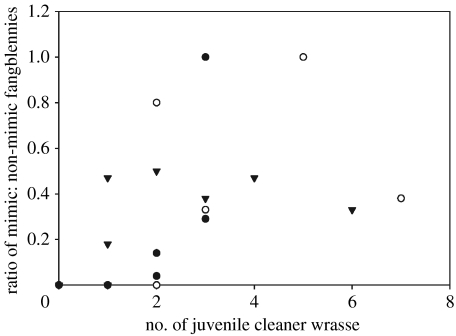
The numbers of mimic fangblennies per swim ranged from 0 to 4; while the total number of mimic and non-mimic fangblennies combined ranged from 0 to 12. In total, 19.4% (34/175) were brown, 46.3% (81/175) olive, 32.0% (56/175) black/blue and 2.3% (4/175) orange. The proportion of mimic to non-mimetic fangblennies increased as the number of juvenile cleaner fish present increased (estimate=0.36; s.e.=0.11, t23=3.26, p=0.003; figure 1). Month, average size and total number of fangblennies were non-significant (p>0.07).
Figure 1.
The ratio of mimic to non-mimic fangblennies present on coral reefs in relation to the number of juvenile cleaner fish present per 20-min swim. Filled circles, April; open circles, July and triangles, January.
(c) Spectrophotometry of fish colours
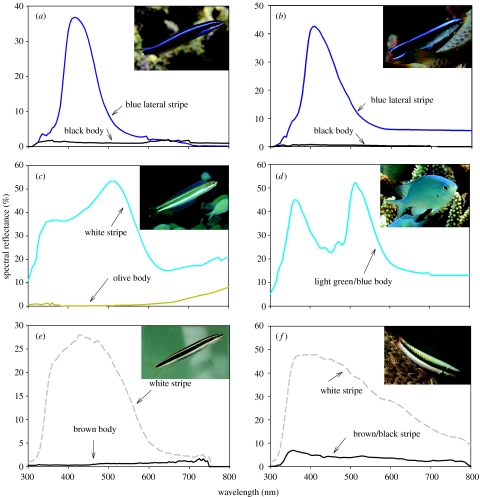
The lateral blue stripes of the juvenile cleaner fish and black/blue mimic fangblennies were very similar with maximum peaks of 415 nm for mimic fangblennies and 410 nm for juvenile cleaner fish (figure 2a,b, respectively).
Figure 2.
Spectral reflectance measurements of (a) mimic black/blue fangblenny, (b) juvenile cleaner fish, (c) olive fangblenny, (d) C. viridis, (e) brown fangblenny and (f) intermediate form of T. amblycephalum. Interestingly, in the mimic colour form the fangblenny does not exhibit a second ventrolateral stripe as shown in the non-mimic colour forms. Grey dashed lines indicate white (or close to white).
Out of 34 fangblennies, nine were found within shoals of other fishes (greater than 20 individuals of same species) on capture. We found 31% (5 out of 16) of olive colour forms associated with blue green chromis Chromis viridis and 43% (three out of seven) of brown forms within shoals of two-tone wrasse Thalassoma amblycephalum (initial phase; Randall et al. 1997), a brown/black and white-coloured fish (figure 2f). Fifty per cent (one out of two) of orange fangblenny was found with Lyretail Anthias (Pseudanthias squamipinnis), an orange-coloured fish (Randall et al. 1997). In each of these pairs, either the body colour or stripe colours of fangblennies appeared to be at least qualitatively similar to the body colour and/or stripes of their ‘shoalmates’. Olive fangblennies (figure 2c) had similar coloured light blue-green stripes compared with the body colour of blue green chromis (figure 2d); however, blue green chromis had a stronger UV (less than 400 nm) component to their body colours compared with fangblennies (figure 2d). The brown fangblenny (figure 2e) had similar body colours, patterns and body shape to T. amblycephalum (figure 2f).
4. Discussion
This is, to our knowledge, the first study to show that a facultative vertebrate mimic rapidly changes to a mimic coloration in the presence of its model. In laboratory experiments, non-mimic bluestriped fangblennies (P. rhinorhynchos) changed to a mimic colour within 30 min when placed in an aquarium tank with its model, a juvenile cleaner fish (L. dimidiatus). This was supported by field observations that showed the proportion of mimic to non-mimic fangblennies was positively related to the number of juvenile cleaner fish in the surrounding environment. Finally, colour analyses showed that mimic fangblennies closely resembled the colours of juvenile cleaner fish; while non-mimic colour forms appeared to be similar to other shoaling fish that they associated with.
Facultative mimicry is a unique strategy that is uncommon in the animal kingdom and allows mimics to resemble a number of different species. This enables individuals to persist in a variety of environmental conditions; for example, such a strategy would be useful to fangblennies on reefs where cleaner fish juveniles are not present, or are present in low numbers. The ability to adopt colour forms different to that of its main model provides the mimic with added strategies for avoiding detection by potential victims of attack, or by potential predators.
Non-mimic fangblennies often hide in similarly coloured shoals of fish (Randall et al. 1997; Côté & Cheney 2005). The similar colour of the stripes of olive fangblennies compared with the body colour of blue green chromis (figure 2d) would help fangblennies to blend in with their shoalmates. Clearly, the body shape of fangblennies is nothing like that of damselfish; however, when hiding in a shoal of fish, only a superficial resemblance to the shoal may be enough to prevent detection by a passing fish or a predator (Crook 1999).
Fish that changed colour were significantly smaller than those that did not change colour, and only small individuals (less than 50 mm) changed colour to the black and blue mimic colour form. Labroides dimidiatus switch from the black and blue juvenile colour form to an adult colour pattern (a white body with a black lateral stripe) at approximately 4–5 cm (Mahon 1994; K.L.Cheney 2006, personal observation); therefore, fangblennies only appear to mimic juvenile cleaner wrasse when they are of a similar size. As fangblennies become larger, the benefits of resembling fishes smaller than themselves may be limited as they become more detectable from their model. Furthermore, fangblennies could potentially lose their ability to change colour once they reach a certain size.
Colour changes in fangblennies did not occur in aquarium experiments when fangblennies were placed with control fishes. However, due to logistical constraints the control fishes we tested were not the same as those we found associated with fangblennies in the field. Therefore, it is possible that only certain species or certain colours may trigger colour changes in non-mimic colour forms. The light environment on coral reefs, which may not have been accurately replicated in our artificial environment, may also affect how colours were perceived by fangblennies (Marshall et al. 2003). For example, the light environment on coral reefs alters with depth, habitat and turbidity (McFarland & Munz 1975; McFarland 1986; Marshall et al. 2003), all of which may affect colour signals that are emitted and thus requires further investigation. However, the signal difference between models and their reef fish mimics appears to be relatively constant with depth and distance (K. L. Cheney & N. J. Marshall 2007, unpublished data).
Colour spectrometry allows us to compare colours based on a non-subjective perspective, and not what we see as humans (Endler 1990). To our knowledge, this is the first time that colour analyses have been used to assess a coral reef fish mimicry system. Fishes have very different visual systems to humans and most reef fish can either have a UV, blue- or green-biased system (Losey et al. 2003; Marshall et al. 2006). While many fishes may block or are unable to detect UV light (Siebeck & Marshall 2001; Losey et al. 2003), those fishes that can detect UV (e.g. many species of damselfish; Losey et al. 2003; Marshall et al. 2006) may be more likely to distinguish, for example, fangblennies from blue green chromis due to the stronger UV component found in chromis colours. A study is currently being conducted to establish how colours in mimicry systems are viewed from different visual perspectives, and in particular examine the differences in the perception of signal differences between model and mimics, using a visual model of colour discrimination thresholds following Vorobyev & Osorio (1998).
Acknowledgments
We would like to thank the staff at Lizard Island Research Station for their logistical support, and to L. Curtis, M. Eckes, B. Cameron and M. Horton for their help in the field. K.L.C., A.S.G. and N.J.M. are supported by the Australian Research Council. They also wish to thank A. Goldizen for comments on the manuscript and S. Blomberg for statistical advice.
References
- Allen G.R, Steene R, Humann P, Deloach N. New World Publications; Jacksonville, FL: 2003. Reef fish identification—tropical pacific. [Google Scholar]
- Armbruster J.W, Page L.M. Convergence of a cryptic saddle pattern in benthic freshwater fishes. Environ. Biol. Fish. 1996;45:249–257. doi:10.1007/BF00003092 [Google Scholar]
- Bennett A.T.D, Cuthill I.C, Partridge J.C, Lunau K. Ultraviolet plumage colors predict mate preferences in starlings. Proc. Natl Acad. Sci. USA. 1997;94:8618–8621. doi: 10.1073/pnas.94.16.8618. doi:10.1073/pnas.94.16.8618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté I.M. Evolution and ecology of cleaning symbioses in the sea. Oceanogr. Mar. Biol. Annu. Rev. 2000;38:311–355. [Google Scholar]
- Côté I.M, Cheney K.L. Distance-dependent costs and benefits of aggressive mimicry in a cleaning symbiosis. Proc. R. Soc. B. 2004;271:2627–2630. doi: 10.1098/rspb.2004.2904. doi:10.1098/rspb.2004.2904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté I.M, Cheney K.L. Choosing when to be a cleaner-fish mimic. Nature. 2005;433:211–212. doi: 10.1038/433211a. doi:10.1038/433211a [DOI] [PubMed] [Google Scholar]
- Cott H.B. Methuen; London, UK: 1940. Adaptive coloration in animals. [Google Scholar]
- Crook A.C. Quantitative evidence for assortative schooling in a coral reef fish. Mar. Ecol. Prog. Ser. 1999;176:17–23. doi:10.3354/meps176017 [Google Scholar]
- Endler, J. A. 1978 A predator's view of animal colour patterns. In Evolutionary biology, vol. 11 (eds M. K. Hecht, W. C. Steere & B. Wallace), pp. 319–364. New York, NY: Plenum Press.
- Endler J.A. On the measurement and classification of colour in studies of animal colour patterns. Biol. J. Linn. Soc. 1990;41:315–352. [Google Scholar]
- Hanlon R.T, Messenger J.B. Adaptive coloration in young cuttlefish (Sepia officinalis)—the morphology and development of body patterns and their relation to behavior. Phil. Trans. R. Soc. B. 1988;320:437–487. doi:10.1098/rstb.1988.0087 [Google Scholar]
- Kuwamara T. Mimicry of the cleaner wrasse Labroides dimidiatus by the blennies Aspidontus taeniatus and Plagiotremus rhynorhynchos. Nanki Seibutu. 1981;23:61–70. [Google Scholar]
- Losey G.S. Crypsis and communication functions of UV–visible coloration in two coral reef damselfish, Dascyllus aruanus and D. reticulatus. Anim. Behav. 2003;66:299–307. doi:10.1006/anbe.2003.2214 [Google Scholar]
- Losey G.S, McFarland W.N, Loew E.R, Zamzow J.P, Nelson P.A, Marshall N.J. Visual biology of Hawaiian coral reef fishes. I. Ocular transmission and visual pigments. Copeia. 2003;2003:433–454. doi:10.1643/01-053 [Google Scholar]
- Lythgoe J.N. Clarendon Press; Oxford, UK: 1979. The ecology of vision. [Google Scholar]
- Mahon J.L. Advantage of flexible juvenile coloration in two species of Labroides (Pisces: Labridae) Copeia. 1994;1994:520–524. doi:10.2307/1447003 [Google Scholar]
- Marshall N.J, Jennings K, McFarland W.N, Loew E.R, Losey G.S. Visual biology of Hawaiian coral reef fishes. III. Environmental light and an integrated approach to the ecology of reef fish vision. Copeia. 2003;2003:467–480. doi:10.1643/01-056 [Google Scholar]
- Marshall N.J, Vorobyev M, Siebeck U.E. What does a reef fish see when it sees a reef fish? Eating nemo. In: Ladich F, Collin S.P, Moller P, Kapoor B.G, editors. Communication in fishes. Visual communication and electric communication. vol. 2. Science Publishers; Enfield, NH: 2006. pp. 393–422. [Google Scholar]
- McCullagh P, Nelder J.A. 2nd edn. Chapman and Hall; London, UK: 1989. Generalized linear models. [Google Scholar]
- McFarland W.N. Light in the sea—correlations with behaviours of fishes and invertebrates. Am. Zool. 1986;26:389–401. [Google Scholar]
- McFarland W.N, Munz F.W. Photic environment of clear tropical seas during the day. 2. Vision Res. 1975;15:1063–1070. doi: 10.1016/0042-6989(75)90002-4. doi:10.1016/0042-6989(75)90002-4 [DOI] [PubMed] [Google Scholar]
- Moland E, Jones G.P. Experimental confirmation of aggressive mimicry by a coral reef fish. Oecologia. 2004;140:676–683. doi: 10.1007/s00442-004-1637-9. doi:10.1007/s00442-004-1637-9 [DOI] [PubMed] [Google Scholar]
- Norman M.D, Finn J, Tregenza T. Dynamic mimicry in an Indo-Malayan octopus. Proc. R. Soc. B. 2001;268:1755–1758. doi: 10.1098/rspb.2001.1708. doi:10.1098/rspb.2001.1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall J.E, Allen G.R, Steene R. Crawford House; Bathurst, Canada: 1997. Fishes of the Great Barrier Reef and Coral sea. [Google Scholar]
- Siebeck U.E. Communication in coral reef fish: the role of ultraviolet colour patterns in damselfish territorial behaviour. Anim. Behav. 2004;68:273–282. doi:10.1016/j.anbehav.2003.11.010 [Google Scholar]
- Siebeck U.E, Marshall N.J. Ocular media transmission of coral reef fish—can coral reef fish see ultraviolet light? Vision Res. 2001;41:133–149. doi: 10.1016/s0042-6989(00)00240-6. doi:10.1016/S0042-6989(00)00240-6 [DOI] [PubMed] [Google Scholar]
- Vorobyev M, Osorio D. Receptor noise as a determinant of colour thresholds. Proc. R. Soc. B. 1998;265:351–358. doi: 10.1098/rspb.1998.0302. doi:10.1098/rspb.1998.0302 [DOI] [PMC free article] [PubMed] [Google Scholar]