Abstract
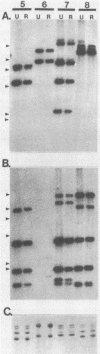
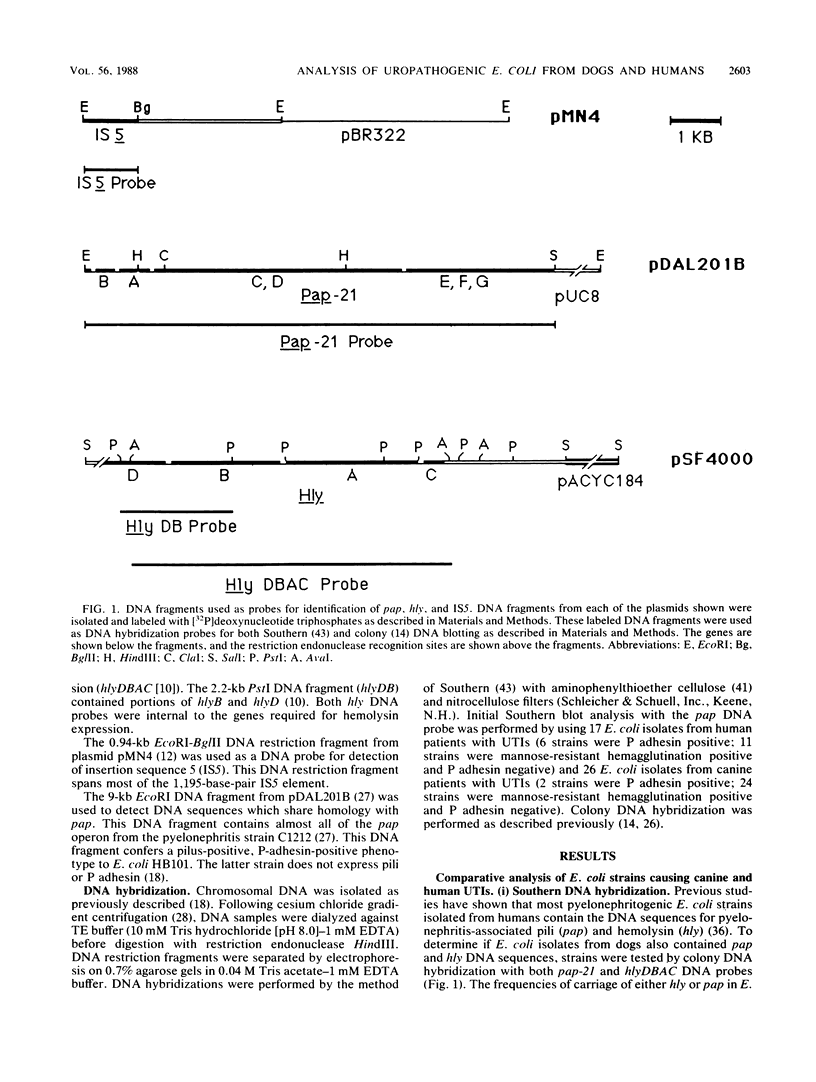
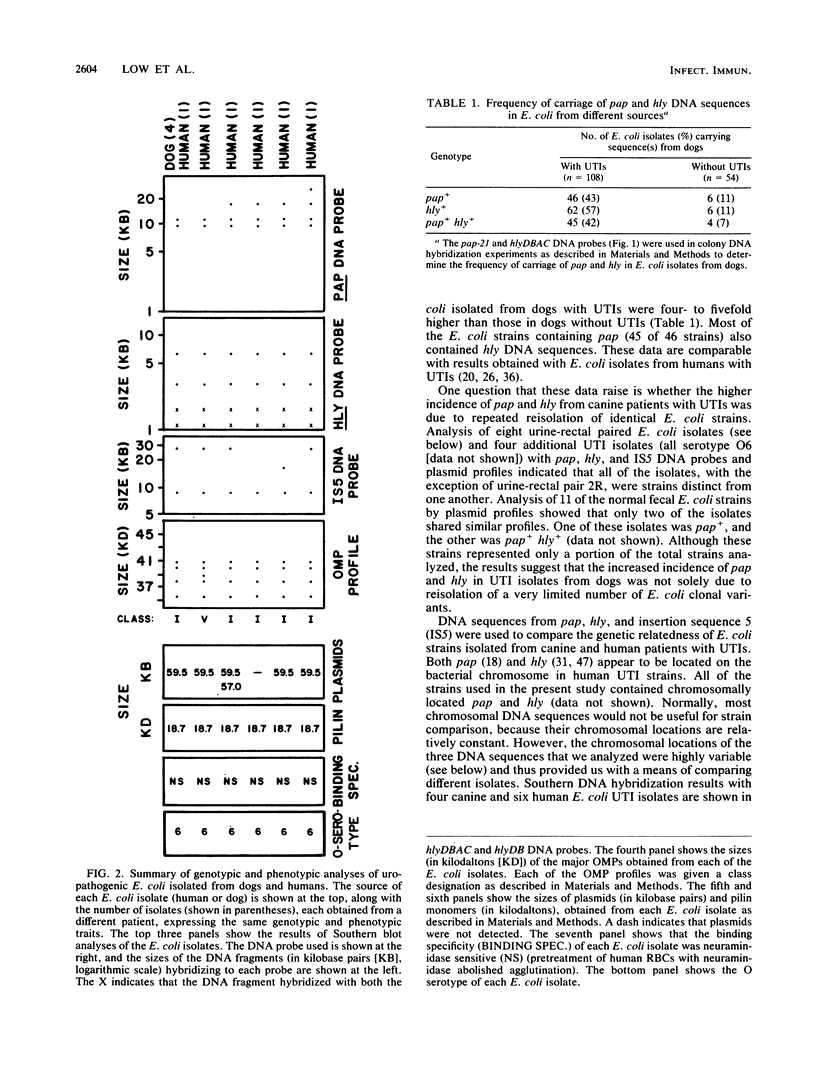
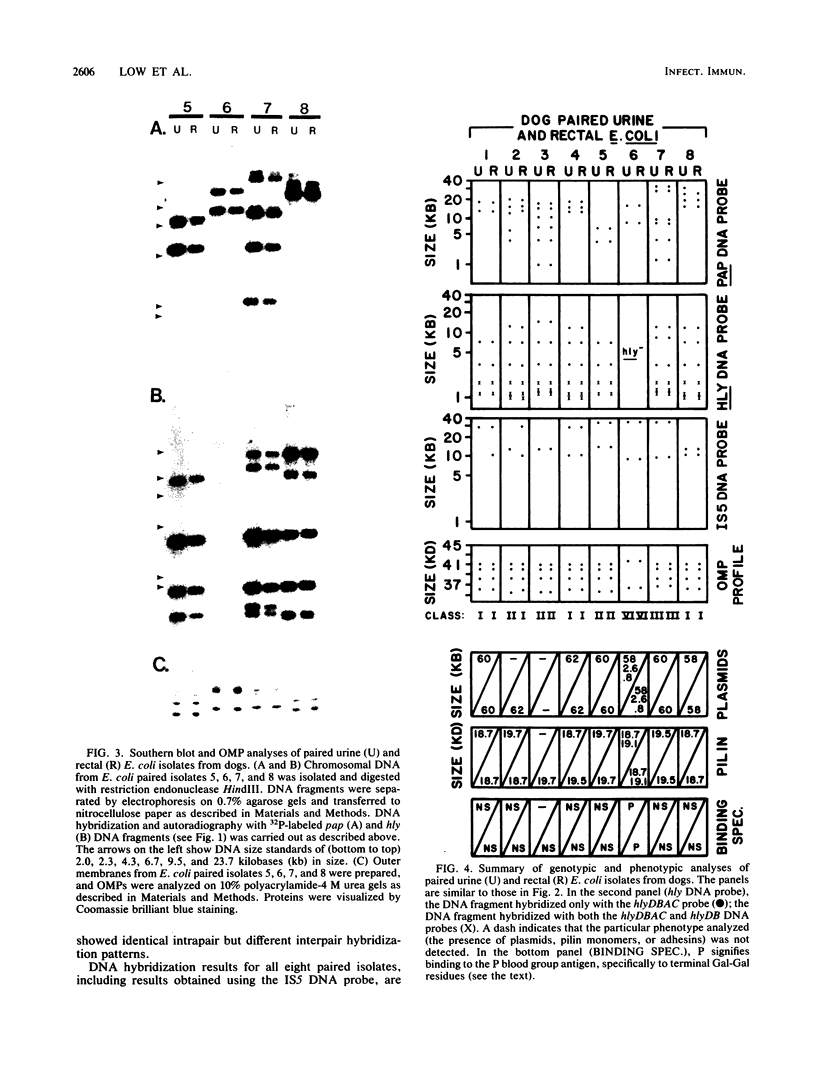
We analyzed Escherichia coli strains isolated from dogs with urinary tract infections (UTIs) in an attempt to determine if any of these strains were similar to E. coli isolated from humans with UTIs. Using genotypic and phenotypic traits, we identified four canine and six human E. coli UTI isolates that all appeared to be closely related or identical. All isolates shared similar DNA sequences for pyelonephritis-associated pili (pap), alpha-hemolysin (hly), and insertion sequence 5 (IS5), on the basis of Southern blot analysis. Similar outer membrane protein, pilin, and plasmid profiles were obtained for each of the isolates, although minor heterogeneity was observed. All of these isolates expressed a neuraminidase-sensitive binding phenotype in contrast to the majority of human isolates, which are known to express an adhesin that recognizes terminal digalactoside residues. Taken together, these results suggest that similar E. coli uropathogens may be capable of infecting both dogs and humans. To determine if the intestinal tracts of dogs were a reservoir for uropathogenic E. coli, eight paired rectal and urine pap+ E. coli strains were cultured from dogs with UTIs. By using the same genotypic and phenotypic criteria described above as a basis for strain identity, seven of eight urine-rectal pairs showed intrapair identity. However, each urine-rectal pair displayed a unique overall profile and could be distinguished from the other pairs. We conclude that the uropathogen colonizing the bladders of dog can also be the predominant strain colonizing the intestinal tracts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham E., Brenner B. E., Simon R. R. Cystitis and pyelonephritis. Ann Emerg Med. 1983 Apr;12(4):228–234. doi: 10.1016/s0196-0644(83)80603-9. [DOI] [PubMed] [Google Scholar]
- Achtman M., Mercer A., Kusecek B., Pohl A., Heuzenroeder M., Aaronson W., Sutton A., Silver R. P. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–335. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achtman M., Pluschke G. Clonal analysis of descent and virulence among selected Escherichia coli. Annu Rev Microbiol. 1986;40:185–210. doi: 10.1146/annurev.mi.40.100186.001153. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Selander R. K. Distribution of multilocus genotypes of Escherichia coli within and between host families. J Hyg (Lond) 1984 Jun;92(3):377–384. doi: 10.1017/s0022172400064597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Selander R. K. Genetic diversity and temporal variation in the E. coli population of a human host. Genetics. 1981 Jul;98(3):467–490. doi: 10.1093/genetics/98.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke E. M., Ewins S., Shooter R. A. Changing faecal population of Escherichia coli in hospital medical patients. Br Med J. 1969 Dec 6;4(5683):593–595. doi: 10.1136/bmj.4.5683.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daifuku R., Stamm W. E. Bacterial adherence to bladder uroepithelial cells in catheter-associated urinary tract infection. N Engl J Med. 1986 May 8;314(19):1208–1213. doi: 10.1056/NEJM198605083141902. [DOI] [PubMed] [Google Scholar]
- Engler J. A., van Bree M. P. The nucleotide sequence and protein-coding capability of the transposable element IS5. Gene. 1981 Aug;14(3):155–163. doi: 10.1016/0378-1119(81)90111-6. [DOI] [PubMed] [Google Scholar]
- Felmlee T., Pellett S., Welch R. A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985 Jul;163(1):94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler J. E., Jr, Stamey T. A. Studies of introital colonization in women with recurrent urinary infections. VII. The role of bacterial adherence. J Urol. 1977 Apr;117(4):472–476. doi: 10.1016/s0022-5347(17)58501-8. [DOI] [PubMed] [Google Scholar]
- Green L., Miller R. D., Dykhuizen D. E., Hartl D. L. Distribution of DNA insertion element IS5 in natural isolates of Escherichia coli. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4500–4504. doi: 10.1073/pnas.81.14.4500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. S., Brinton C. C., Jr Identification and characterization of E. coli type-1 pilus tip adhesion protein. Nature. 1988 Mar 17;332(6161):265–268. doi: 10.1038/332265a0. [DOI] [PubMed] [Google Scholar]
- Hartl D. L., Dykhuizen D. E. The population genetics of Escherichia coli. Annu Rev Genet. 1984;18:31–68. doi: 10.1146/annurev.ge.18.120184.000335. [DOI] [PubMed] [Google Scholar]
- Hull R. A., Gill R. E., Hsu P., Minshew B. H., Falkow S. Construction and expression of recombinant plasmids encoding type 1 or D-mannose-resistant pili from a urinary tract infection Escherichia coli isolate. Infect Immun. 1981 Sep;33(3):933–938. doi: 10.1128/iai.33.3.933-938.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John J. F., Jr, Twitty J. A. Plasmids as epidemiologic markers in nosocomial gram-negative bacilli: experience at a university and review of the literature. Rev Infect Dis. 1986 Sep-Oct;8(5):693–704. doi: 10.1093/clinids/8.5.693. [DOI] [PubMed] [Google Scholar]
- Knapp S., Hacker J., Then I., Müller D., Goebel W. Multiple copies of hemolysin genes and associated sequences in the chromosomes of uropathogenic Escherichia coli strains. J Bacteriol. 1984 Sep;159(3):1027–1033. doi: 10.1128/jb.159.3.1027-1033.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Saxén H., Hultberg H., Svenson S. B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982 Jul;37(1):286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ling G. V., Biberstein E. L., Hirsh D. C. Bacterial pathogens associated with urinary tract infections. Vet Clin North Am Small Anim Pract. 1980 Nov;9(4):617–630. doi: 10.1016/s0195-5616(79)50077-1. [DOI] [PubMed] [Google Scholar]
- Low D., David V., Lark D., Schoolnik G., Falkow S. Gene clusters governing the production of hemolysin and mannose-resistant hemagglutination are closely linked in Escherichia coli serotype O4 and O6 isolates from urinary tract infections. Infect Immun. 1984 Jan;43(1):353–358. doi: 10.1128/iai.43.1.353-358.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low D., Robinson E. N., Jr, McGee Z. A., Falkow S. The frequency of expression of pyelonephritis-associated pili is under regulatory control. Mol Microbiol. 1987 Nov;1(3):335–346. doi: 10.1111/j.1365-2958.1987.tb01940.x. [DOI] [PubMed] [Google Scholar]
- Moch T., Hoschützky H., Hacker J., Kröncke K. D., Jann K. Isolation and characterization of the alpha-sialyl-beta-2,3-galactosyl-specific adhesin from fimbriated Escherichia coli. Proc Natl Acad Sci U S A. 1987 May;84(10):3462–3466. doi: 10.1073/pnas.84.10.3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller D., Hughes C., Goebel W. Relationship between plasmid and chromosomal hemolysin determinants of Escherichia coli. J Bacteriol. 1983 Feb;153(2):846–851. doi: 10.1128/jb.153.2.846-851.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norgren M., Normark S., Lark D., O'Hanley P., Schoolnik G., Falkow S., Svanborg-Edén C., Båga M., Uhlin B. E. Mutations in E coli cistrons affecting adhesion to human cells do not abolish Pap pili fiber formation. EMBO J. 1984 May;3(5):1159–1165. doi: 10.1002/j.1460-2075.1984.tb01945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Lark D., Hull R., Norgren M., Båga M., O'Hanley P., Schoolnik G., Falkow S. Genetics of digalactoside-binding adhesin from a uropathogenic Escherichia coli strain. Infect Immun. 1983 Sep;41(3):942–949. doi: 10.1128/iai.41.3.942-949.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Falkow S., Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985 Feb;75(2):347–360. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Normark S., Falkow S., Schoolnik G. K. Mannose-sensitive and Gal-Gal binding Escherichia coli pili from recombinant strains. Chemical, functional, and serological properties. J Exp Med. 1983 Nov 1;158(5):1713–1719. doi: 10.1084/jem.158.5.1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Low D., Romero I., Lark D., Vosti K., Falkow S., Schoolnik G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med. 1985 Aug 15;313(7):414–420. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Birch-Andersen A., Kanamori M., Svanborg-Edén C. O, K, H and fimbrial antigens in Escherichia coli serotypes associated with pyelonephritis and cystitis. Scand J Infect Dis Suppl. 1982;33:18–25. [PubMed] [Google Scholar]
- Portnoy D. A., Falkow S. Virulence-associated plasmids from Yersinia enterocolitica and Yersinia pestis. J Bacteriol. 1981 Dec;148(3):877–883. doi: 10.1128/jb.148.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roberts A. P., Linton J. D., Waterman A. M., Gower P. E., Koutsaimanis K. G. Urinary and faecal Escherichia coli O-sero-groups in symptomatic urinary-tract infection and asymptomatic bacteriuria. J Med Microbiol. 1975 May;8(2):311–318. doi: 10.1099/00222615-8-2-311. [DOI] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stamey T. A., Timothy M., Millar M., Mihara G. Recurrent urinary infections in adult women. The role of introital enterobacteria. Calif Med. 1971 Jul;115(1):1–19. [PMC free article] [PubMed] [Google Scholar]
- VOSTI K. L., GOLDBERG L. M., MONTO A. S., RANTZ L. A. HOST-PARASITE INTERACTION IN PATIENTS WITH INFECTIONS DUE TO ESCHERICHIA COLI. I. THE SEROGROUPING OF E. COLI FROM INTESTINAL AND EXTRAINTESTINAL SOURCES. J Clin Invest. 1964 Dec;43:2377–2385. doi: 10.1172/JCI105112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen V., Korhonen T. K., Jokinen M., Gahmberg C. G., Ehnholm C. Blood group M specific haemagglutinin in pyelonephritogenic Escherichia coli. Lancet. 1982 May 22;1(8282):1192–1192. doi: 10.1016/s0140-6736(82)92264-4. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Dellinger E. P., Minshew B., Falkow S. Haemolysin contributes to virulence of extra-intestinal E. coli infections. Nature. 1981 Dec 17;294(5842):665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- Welch R. A., Hull R., Falkow S. Molecular cloning and physical characterization of a chromosomal hemolysin from Escherichia coli. Infect Immun. 1983 Oct;42(1):178–186. doi: 10.1128/iai.42.1.178-186.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerlund B., Pere A., Korhonen T. K., Jarvinen A. K., Siitonen A., Williams P. H. Characterisation of Escherichia coli strains associated with canine urinary tract infections. Res Vet Sci. 1987 May;42(3):404–406. [PubMed] [Google Scholar]