Abstract
Animals can use socially transmitted information to learn about the distribution and quality of resources without incurring the costs associated with having to search for and sample them first hand. Recently, it has been shown that the use of chemical social information specific to patterns of diet and habitat use is an important mechanism underpinning recognition and social organization in shoaling fishes. In this study we revealed that the use of resource-specific chemical information is not limited to conspecifics, or even members of the same taxon. In a series of laboratory experiments, we showed that threespine sticklebacks (Gasterosteus aculeatus) could recognize similar patterns of habitat use in common prawns (Leander serratus), preferentially orientating towards groups of prawns exposed to the same habitats as themselves, and even selecting foraging patches located close to them. Prawns were seen to use habitat-specific cues generated by conspecifics, but not by sticklebacks, suggesting that the benefits of forming these heterospecific social association patterns may be unequal for prawns and fishes. Our findings suggest that some species might use co-occurring, unrelated species as information centres in order to orient and locate resources within their surroundings.
Keywords: social information, social learning, interspecific interactions, groups, shoals, flocks
1. Introduction
Animals can collect information about their environment privately, allowing them to acquire accurate and up to date information about their surroundings, but at the cost of expended sampling effort and potential exposure to heightened predation risk. Alternatively, they can use socially transmitted information, which may often be less accurate, but which allows them to minimize many of the costs associated with gathering information first hand. Such a strategy can potentially enable them to navigate with greater efficiency, to detect and evade predators more effectively, or to more quickly locate and gauge the quality of resources (Laland 2004; Kendal et al. 2005). In nature mixed-species groups occur frequently (Krause & Ruxton 2002) and unsurprisingly it has been shown that the use of social information is not restricted to interactions between conspecifics. Interspecific social information use has been documented in a range of mixed-species assemblages including passerine (Dolby & Grubb 1998, 1999, 2000) and wading birds (Caldwell 1981), fishes (Coolen et al. 2003) and primates (Peres 1993). Indeed, social information use may be the primary benefit gained by some constituents of mixed-species groups, allowing them to exploit the greater ability of their heterospecific groupmates to locate prey resources or detect approaching predators.
Recently, it has been shown that fishes use a type of social information based upon habitat- and diet-specific chemical cues. These are accrued and released following exposure to a particular habitat or prey type, and can be detected by others, with individuals being seen to preferentially shoal with conspecifics and closely related heterospecifics that share similar patterns of resource use to themselves (Ward et al. 2004, 2005, 2007; Webster et al. in press). It is thought that doing so could serve two primary purposes. First, it may form the basis of a producer–scrounger relationship; by matching patterns of resource use, the joining individual gains the opportunity to learn about prey distribution and quality from its prospective shoalmates, or even to kleptoparasitize their prey captures. Second, it may aid navigation, with fishes using cues emanating from their shoalmates alongside information obtained through asocial interactions with the environment to determine both their own location and the distribution of resources. Water is a highly effective solvent and medium for many volatile compounds that can potentially be detected and used as cues by aquatic animals, and water chemistry, influenced by such factors as salinity, substrate materials, vegetation growth and terrestrial run-off, can vary locally and at very fine spatial scales. Recent work has revealed that wild fishes use habitat-specific cues to discriminate between conspecifics from spatially close but chemically distinct areas of habitat in nature (Ward et al. 2007). These cues might be especially useful to fishes, which show low shoal fidelity, but high site fidelity. Given this, we might expect to see that fishes use habitat-specific cues generated by members of heterospecific species that they encounter in addition to those produced by their shoalmates. This idea forms the premise of the following investigation.
In a laboratory study, we investigated patterns of intra- and interspecific use of habitat-specific chemical cues by naturally co-occurring fishes and decapod crustaceans, threespine sticklebacks (Gasterosteus aculeatus) and common prawns (Leander serratus). These species are found together in shallow estuarine waters containing vegetation or other cover, which provide shelter from flowing water and refuge from predators. Both are generalists, foraging for microinvertebrates, with the prawn also feeding upon vegetable matter (Forster 1951; Bell & Foster 1994). Thus, these species possess overlapping habitat and prey resource use patterns, and we suggest that they should therefore make use of social information derived from chemical cues relating to these.
Our study contained three parts. In part 1 we investigated shoaling tendencies when all individuals had been exposed to the same habitat treatment. We predicted that both species should preferentially join larger groups, a generally adaptive response to predation risk, which is likely to be intense in their natural habitat. In part 2 we examined the use of habitat-specific chemical cues by focal animals. We predicted that sticklebacks and prawns would preferentially join both con- and heterospecific stimulus groups that had been exposed to similar habitat condition treatments as themselves, since doing so could potentially provide them with information relating to orientation and resource distribution (Ward et al. 2007; Webster et al. in press). Finally, in part 3 we tested the prediction that group choice decisions mediated by habitat-specific cues should indirectly influence prey patch choice. Specifically, we predicted that sticklebacks would spend more time foraging at a prey patch situated close to stimulus prawns previously exposed to the same habitat as themselves than they would at an otherwise identical prey patch located closer to stimulus prawns that had been exposed to a different habitat.
2. Material and methods
(a) Study system
Threespine sticklebacks measuring 25–35 mm in length and common prawns measuring 30–40 mm long were collected from the estuary of the Great Eau, a semi-natural drainage network in Lincolnshire, UK, using dipnets at low tide. In the laboratory they were transferred to 25 l aquaria, with each species held separately at a density of 20 animals per tank. The specific gravity of the tankwater was held at 1.008, the temperature at 10°C with a light : dark regime of 12 : 12 hours. Water was aerated and 50% water changes were performed three times per week. The prawns were fed daily on frozen bloodworm, Daphnia and flake fish food, while the sticklebacks received bloodworm and Daphnia only. They were held under these conditions for eight weeks before the experiments began. We conducted three experiments, using a binary choice assay to investigate the social preferences of the study animals.
(b) Binary choice tank and testing procedure
Binary choice tests were performed in an experimental tank described in figure 1. Within each trial, the focal and stimulus animals were size matched by standard length to within less than 1 mm of each other. A control experiment described below and in §3 revealed that neither species could use habitat-specific cues contained in the water alone, in the absence of stimulus conspecifics. As an extra precaution, before being added to the test tank, stimulus animals were first placed in a 12 l tank of freshwater and held there for 2 min. This reduced the possibility of water from their holding tanks being transferred to the test tank on the stimulus animal, giving us confidence that any chemical cues affecting the behaviour of the focal animal arose from the stimulus animals themselves. The number of stimulus animals added to each stimulus chamber and the experimental treatments to which they were subjected are described in parts 1 to 3. The positions of the stimulus animals were varied between trials in order to control for tank-end bias. Stimulus animals were allowed to settle in the test tank for 5 min before each trial. Dye tests revealed that this settling period was sufficient for water to flow through the perforated wall of the stimulus chamber and spread through the section of the tank holding the focal animal. A single focal animal was then selected and was placed within a 7 cm×7 cm×22 cm tall holding unit constructed from the same perforated material as the stimulus compartment screens. The holding unit was situated in the centre of the test tank. The focal animal was held within the holding unit for 5 min, during which it could assimilate visual and chemical cues from the stimulus animals. The holding unit was then removed and the focal animal released, beginning the trial. The trial duration was 5 min and we recorded the total amount of time the focal animal spent in the association zone in front of either stimulus compartment. Following each trial, we added new stimulus animals to the second test tank. While these were settling for 5 min, we changed the water in the first test tank to prevent habitat cues from accumulating between trials.
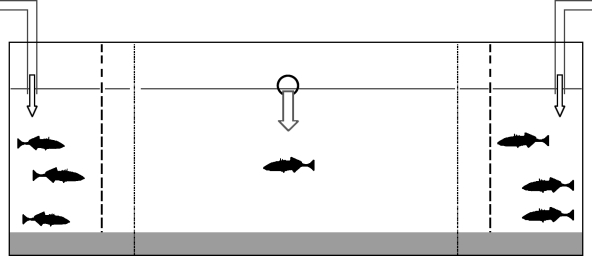
Figure 1.
The binary choice tank used in parts 1–3 of this study measured 45 cm×17 cm×18 cm deep with a water depth of 15 cm. At each end of the tank along its longest axis was an 8 cm wide stimulus chamber, separated from the central section of the tank by screens of colourless perforated plastic (perforation diameter 0.1 cm, 5±1 perforations cm−2). This allowed the exchange of both visual and chemical cues. A 2 cm deep substrate of coarse sand was provided in the central section of the tank and in the two stimulus chambers. On the outside of the glass, we marked two association zones, indicated by vertical black lines, 2 cm from each of the stimulus chambers. This distance falls well within the range of inter-individual distances seen in free-ranging fish shoals (Pitcher & Parrish 1993). The number of stimulus animals added to each stimulus chamber and the experimental treatments to which they were subjected are described in §2d–f. The experimental tank contained fresh water obtained from the recirculating laboratory supply. Water from the same source was pumped into the centre of each of the two stimulus chambers at a rate of 20 cm3 min−1 and allowed to drain out of an overflow outlet located at the waterline at the centre of the rear wall of the tank. This served to carry chemical cues from stimulus animals from either compartment into the central section of the tank where the focal animal was held. Two test tanks were set up and used alternately between trials.
Binary choice tests generate data with low statistical power. In order to avoid making type II statistical errors, we performed addition replicates in those experiments where we initially failed to detect any differences in the association preferences of the study animals. For this reason, the number of replicates differs between experiments.
(c) Control experiment for use of asocial habitat-specific cues
We performed a control experiment in order to determine whether prawns or sticklebacks could use habitat-specific cues contained in the water, in the absence of conspecific demonstrators. These were performed as described above and in figure 1, except that no stimulus demonstrators were present in the stimulus chambers. Sticklebacks and prawns were conditioned to either the saline or the vegetated habitat treatments, as described in part 2, below. Water from the two simulated habitats was pumped into the test tank via the two stimulus chambers (figure 1). One received water from the vegetated treatment, while the other received from the saline treatment. At no time had any stimulus animals been housed in this water. We tested a total of 30 sticklebacks and 28 prawns, with half-conditioned to each habitat treatment, using the procedure detailed above.
(d) Part 1. Social aggregation
Though not a prerequisite, the tendency to form cohesive groups with conspecifics can enhance the potential for an individual to detect and use social information. In this component of the study, we sought to determine the tendency of each species to aggregate, using the binary choice apparatus and procedure described below. All focal animals were exposed to the same habitat treatment, water of specific gravity 1.008, and all were housed individually in chemically and visually isolated 12 l tanks containing aerated water for 6 hours prior to testing. Stimulus animals were drawn at random directly from stock-holding tanks. Within each trial, all focal and stimulus animals originated from different holding tanks, in order to control for any effects of familiarity, and all were size matched to within less than 1 mm body length in order to control for size assortive shoaling preferences. Experimental animals were not fed for 36 hours prior to being tested and no animal was used more than once. We performed the following six experiments:
A focal stickleback was given the choice between two stimulus compartments containing six and zero sticklebacks, respectively (n=12 replicates).
A focal stickleback was given the choice between two stimulus compartments containing four and two sticklebacks, respectively (n=12 replicates).
A focal prawn was given the choice between two stimulus compartments containing six and zero prawns, respectively (n=18 replicates).
A focal prawn was given the choice between two stimulus compartments containing four and two prawns, respectively (n=18 replicates).
The final two experiments considered interspecific association preferences.
A focal stickleback was given the choice between two stimulus compartments containing six and zero prawns, respectively (n=45 replicates).
A focal prawn was given the choice between two stimulus compartments containing six and zero sticklebacks, respectively (n=18 replicates).
(e) Part 2. Group choice and habitat-specific chemical cues
Previous research has revealed that sticklebacks preferentially shoal with conspecifics that have recently occupied similar habitats to themselves (Ward et al. 2004, 2005, 2007; Webster et al. in press). Here, we sought to determine whether these cues influenced heterospecific group preferences. A focal animal was given the choice of shoaling with a stimulus group that had been exposed to the same habitat treatment as itself or with a group that had been exposed to an alternative habitat. We used two habitat treatments referred to here as vegetated and saline. The vegetated treatment simulated a freshwater habitat unit with high concentrations of tannins, characteristic of the heavily vegetated natural river channel and man-made drainage ditches that connect to the largely unvegetated estuary of the Great Eau. We replicated these habitat conditions by using 0.5 ml l−1 of a purpose designed solution (Blackwater Extract, Tetra GmbH, Herrenteich 78, 49324 Melle, Germany). The saline treatment consisted of saline water with a specific gravity of 1.016 and simulated brackish areas of habitat free from decaying organic matter. Salinity varies locally in the estuary of the Great Eau, under the influence of both the tidal cycle and the run-off from connected saltwater ditches and saline to hypersaline marsh pools. Our simulated habitat types were therefore representative of those occurring naturally within the drainage basin, giving our experimental design ecological relevance. This protocol is adapted from that used by Webster et al. (in press).
Focal animals were conditioned individually to either the saline or the vegetated treatments, while stimulus animals were conditioned in groups of three. Within trials, no two stimulus animals were taken from the same conditioning tank. Conditioning took place in aerated 12 l aquaria that were visually and chemically isolated from each other, and test animals were conditioned for 6 hours at 10°C. All experimental animals had been deprived of food for 36 hours prior to the beginning of the experiment. Focal animals were given a binary choice between shoaling with stimulus groups of three animals each from either their own or the alternative habitat, using the binary choice procedure described below. No animal was used more than once. We performed the following four experiments:
A stickleback was given the choice of shoaling with stimulus conspecifics from its own or the alternative habitat (n=12 replicates, six with the focal fishes conditioned to the saline habitat and six with it conditioned to the vegetated habitat).
A prawn was given the choice of grouping with stimulus conspecifics from its own or the alternative habitat (n=24 replicates, 12 with the focal prawn conditioned to the saline habitat and 12 with it conditioned to the vegetated habitat).
A stickleback was given the choice of shoaling with stimulus prawns from its own or from the alternative habitat (n=24 replicates, 12 with the focal fishes conditioned to the saline habitat and 12 with it conditioned to the vegetated habitat).
A prawn was given the choice of grouping with stimulus sticklebacks from its own or the alternative habitat (n=49 replicates, 25 with the focal prawn conditioned to the saline habitat and 24 with it conditioned to the vegetated habitat).
(f) Part 3. Local enhancement: do sticklebacks use habitat-specific cues generated by prawns to select between prey patches?
Self-referent matching of habitat-specific cues may represent a mechanism by which animals navigate within their habitats, potentially influencing how they use spatially discreet resources. Here, we sought to determine whether the habitat-specific cues generated by prawns could determine prey patch use by the sticklebacks receiving them. This experiment used a modified version of the binary choice experiment described above and in figure 1. Immediately in front of each of the stimulus chambers, we placed a container measuring 2 cm×3 cm×5 cm height. Each container held fresh water and 50 live Daphnia. Each was clear except for the back wall, which was black. These containers were sealed so that visual, but not chemical, cues were available to the focal fishes, while neither visual nor chemical cues could be detected by the stimulus prawns. Each container represented a spatially discreet prey patch. Sticklebacks and prawns were conditioned to the two habitat-type treatments, saline and vegetated, as described above in part 2. A stickleback was then given the choice of shoaling with stimulus prawns from its own or the alternative habitat, and in addition to recording the time that it spent in front of either stimulus compartment, we also recorded the number of strikes it made against the Daphnia in either container, taking these data as indicators of foraging effort allocation. Sixteen replicates were performed, eight with the focal fishes conditioned to the saline habitat and eight with it conditioned to the vegetated habitat.
(g) Statistical analyses of binary choice data
For part 1 we subtracted the proportion of time spent by the focal animal shoaling with the numerically smaller stimulus group from that spent shoaling with the larger group. In parts 2 and 3 we subtracted the proportion of time it spent shoaling with the stimulus group that had been exposed to the alternative habitat from which it spent with the stimulus group that had been exposed to the same habitat as itself. These difference values were compared against a null expected value of zero using Wilcoxon signed-rank tests. In part 3, the numbers of strikes made by the focal fishes against the Daphnia held in front of the group of prawns from the same habitat as itself were subtracted from the numbers of strikes made against the Daphnia in front of the alternative stimulus group, and compared to a null expected value of zero, again using a Wilcoxon signed-rank test.
3. Results
(a) Control experiment for use of asocial habitat-specific cues
Neither sticklebacks nor prawns displayed any preference for the end of the tank receiving water from the habitat treatment in which they had previously been housed over the end receiving water from the alternative habitat treatment (proportion of trial time ±s.e.: sticklebacks: 0.49±0.07 versus 0.50±0.07, Wilcoxon signed-rank test: n=30, Z=−0.11, p=0.93; prawns: 0.44±0.05 versus 0.55±0.06, Wilcoxon signed-rank test: n=28, Z=−1.09, p=0.23). These findings suggest that the data presented below in parts 2 and 3 reflect responses of the focal animals to socially transmitted habitat cues, rather than responses to any residual cues in the water that might have been transferred into the test tank when the stimulus animals were added.
(b) Part 1. Social aggregation
Both species showed a significant preference for joining larger groups of conspecifics (Wilcoxon signed-rank test: sticklebacks, group sizes 6 versus 0: n=12, Z=−4.22, p<0.001; group sizes 4 versus 2: n=12, Z=−3.69, p<0.001; prawns, group sizes 6 versus 0: n=18, Z=−3.42, p=0.001; group sizes 4 versus 2: n=18, Z=−4.82, p<0.001). When we tested heterospecific shoaling preferences, we saw that sticklebacks given a choice between stimulus chambers containing six and zero prawns showed no association preference for either (n=45, Z=−1.21, p=0.21), while prawns given a choice between stimulus chambers containing six and zero sticklebacks avoided the sticklebacks, spending significantly more time near the empty chamber (n=18, Z=−3.38, p=0.001; figure 2).
Figure 2.
The mean difference in proportional trial time (±s.e.) spent by focal sticklebacks or prawns grouping with different stimulus con- or heterospecifics. In each instance, the time spent with the numerically smaller group has been subtracted from the time spent with the larger group. As such, a positive score indicates a preference for the larger group, a negative score indicates a preference for the smaller group and a score of zero indicates no preference for either. *p<0.05; n.s., not significantly different from 0.
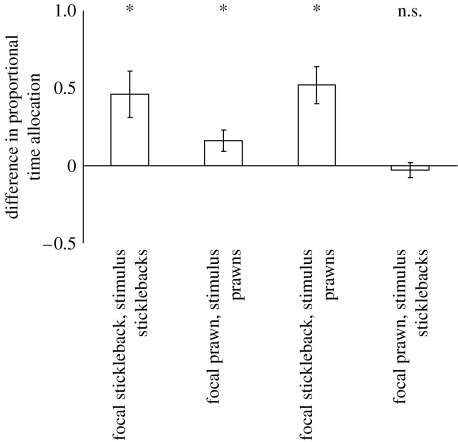
(c) Part 2. Group choice and habitat-specific chemical cues
Both species preferentially grouped with stimulus conspecifics that had been exposed to the same habitat treatment as themselves (Wilcoxon signed-rank test: sticklebacks, n=24, Z=−2.33, p=0.011; prawns, n=24, Z=−1.19, p=0.046). Focal sticklebacks spent significantly more time close to prawns that had been exposed to the same habitat treatment as themselves than they did close to prawns that had been exposed to the alternative habitat treatment (n=24, Z=−2.95, p=0.003). Focal prawns, however, showed no preference for associating with stimulus sticklebacks exposed to either the same or the alternative habitat treatment to their own (n=49, Z=−0.45, p=0.64; figure 3).
Figure 3.
The mean difference in proportional trial time (±s.e.) spent by focal sticklebacks or prawns grouping with stimulus con- or heterospecifics that had been exposed to the same habitat treatment to themselves or to an alternative habitat treatment. In each instance, the time spent with the group that was exposed to the alternative habitat treatment has been subtracted from the time spent with the group exposed to the same habitat treatment. As such, a positive score indicates a preference for the group exposed to the same habitat treatment, a negative score indicates a preference for the group that was exposed to the alternative habitat treatment and a score of zero indicates no preference for either. *p<0.05; n.s., not significantly different from 0.
(d) Part 3. Patch choice: do sticklebacks use habitat-specific cues generated by prawns to select between prey patches?
As observed in part 2, the focal sticklebacks in this experiment spent significantly more time close to the prawns that had been exposed to the same habitat treatment as themselves than they did close to the prawns that had been exposed to the alternative habitat treatment (proportion of trial time ±s.e.: 0.60±0.09 versus 0.27±0.09, Wilcoxon signed-rank test: n=16, Z=−2.50, p=0.019). They also allocated more foraging effort (inferred from the number of strikes directed against the Daphnia within the prey containers) to the prey patch located next to the stimulus prawns from the same habitat treatment as themselves than they did to the prey patch located next to alternative habitat stimulus group (mean strike rate per minute ±s.e.: 1.8±0.6 versus 0.5±0.3, Wilcoxon signed-rank test: n=16, Z=−2.99, p=0.010). This effect is probably related to fish spending more time in proximity to the prawns from the same habitat treatment, rather than an increase in foraging rate per se.
4. Discussion
Mixed-species groups occur frequently in nature (Krause & Ruxton 2002), arising both passively, when different species come together to exploit resources within a given area at the same time, and actively when individuals are drawn to and preferentially associate with heterospecifics. The findings of part 1 of our study suggest that any aggregations of sticklebacks and prawns in nature probably arise passively as a result of shared habitat preferences, since we saw no evidence of interspecific social attraction when both species had been exposed to the same freshwater habitat treatment, and were tested in ‘neutral’ water obtained from the same source. Both species showed strong preferences for joining numerically larger groups of conspecifics; however, sticklebacks exhibited no tendency to group with prawns, while the prawns actually avoided the stimulus group of sticklebacks. This latter finding implies that prawns may experience costs by associating with sticklebacks, perhaps because sticklebacks out compete them for prey. Interestingly, despite showing no preference for grouping with prawns based upon group size in part 1, in part 2, where the stimulus groups had been held under different habitat treatments, we saw that sticklebacks were attracted to stimulus groups of prawns that had been exposed to the same habitat treatment as themselves. Furthermore, part 3 of our study revealed that association preferences arising from the use of habitat-specific cues generated by focal prawns also influenced the foraging patch choice of sticklebacks. Here, we saw that focal sticklebacks made more foraging strikes against prey located next to stimulus groups of prawns that had been exposed to the same habitat treatment as themselves, an effect that was probably due to the fishes spending more time in that location. Previous research has demonstrated social organization based upon self-referent matching of resource-specific cues in fishes (Ward et al. 2004, 2005, 2007; Webster et al. in press). Accordingly, we saw here that sticklebacks and prawns preferentially joined conspecific stimulus groups that had been exposed to the same habitat treatment as themselves, supporting the findings of these earlier studies, and also revealing that a similar form of social organization operates in social crustaceans.
Individuals that group with others that are exploiting the same resources are subject to both costs and benefits. Resource competition is likely to be a major cost, since both the joining individual and its prospective group mates will be exploiting the same, probably finite resource. Weighted against this cost are the potential benefits that might be derived from gathering social information from the group. This might include cheap information about the quality and distribution of local resources. Related to this, it has been suggested that resource use matching might facilitate orientation, since the cues that fishes assimilate from their surroundings might be specific to particular locations or habitat types within their home ranges, producing information that others can use to navigate within their social environment (Ward et al. 2007).
Why should sticklebacks actively group with prawns that have been exposed to the same habitat conditions as themselves? An animal is able to draw upon a number of information sources as it navigates within its environment. These may include learned spatial maps (Odling-Smee & Braithwaite 2003) and experience-based associations between habitat characteristics and resource distribution (Webster & Hart 2004, 2006). More generally, they can acquire and update fresh information through asocial interactions with the environment and, also socially, through observation and interaction with others (Brown & Laland 2003). Matching of resource-specific cues may be used in conjunction with these other sources of information to provide fishes with a comprehensive array of information about their surroundings. In turbid aquatic environments where the use of vision is limited, animals could orientate using predictable differences in odour profiles as olfactory landmarks, alongside, or even in place of, visual ones.
By consuming prey and assimilating water-borne cues from their habitat, aquatic animals can concentrate these cues, acting as beacons exuding chemical information to others in the vicinity. This effect may be especially important when background levels of these chemical cues are low, or when they are dispersed by water currents, making it difficult to detect them directly from the environment. Thus, it could be adaptive for fishes to not only choose between groups of conspecifics on the basis of such cues but also move towards groups of heterospecifics that are producing similar cues. Doing so may enable them to gather and use chemical social information as a proxy for resource distribution, local habitat characteristics or even their location relative to different habitat components.
Existing research has revealed that resource-specific social information derived from habitat and diet cues plays a significant role in the social organization of fish shoals and the structuring of single- and mixed-species groups. Future work could build upon these findings in a number of ways. The trade-offs associated with social organization based upon resource use matching, specifically the costs of grouping with direct competitors versus the benefits of potential access to social information merit investigation. It would also be useful to determine how individuals balance these costs and benefits under the influence of factors, such as hunger, previous experience and uncertainty about resource distribution. Finally, by influencing association preferences and social interactions, resource use matching might facilitate non-random patterns of information transmission by giving rise to short-term social networks within populations, something that is also worthy of further attention.
Acknowledgments
Experiments were carried out at the Biology Department of the University of Leicester. M.M.W. was funded by a NERC Studentship. We thank Prof. Anne Magurran and two anonymous referees for their helpful comments on an earlier version of this manuscript. All experimental procedures in this study adhered to the current laws of the United Kingdom.
References
- Bell M.A, Foster S.A. Oxford Science Publications; Oxford, UK: 1994. The evolutionary biology of the threespine stickleback. [Google Scholar]
- Brown C, Laland K.N. Social learning in fishes: a review. Fish Fish. 2003;4:280–288. [Google Scholar]
- Caldwell G.S. Attraction to tropical mixed-species heron flocks—proximate mechanism and consequences. Behav. Ecol. Sociobiol. 1981;8:99–103. doi:10.1007/BF00300821 [Google Scholar]
- Coolen I, van Bergen Y, Day R.L, Laland K.N. Heterospecific use of public information by fish in a foraging context. Proc. R. Soc. B. 2003;270:2413–2419. doi: 10.1098/rspb.2003.2525. doi:10.1098/rspb.2003.2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolby A.S, Grubb T.C., Jr. Benefits to satellite members in mixed-species foraging groups: an experimental analysis. Animal Behaviour. 1998;56:501–509. doi: 10.1006/anbe.1998.0808. doi:10.1006/anbe.1998.0808 [DOI] [PubMed] [Google Scholar]
- Dolby A.S, Grubb T.C. Functional roles in mixed-species foraging flocks: A field manipulation. Auk. 1999;116:557–559. [Google Scholar]
- Dolby A.S, Grubb T.C. Social context affects risk taking by a satellite species in a mixed-species foraging group. Behav. Ecol. 2000;11:110–114. doi:10.1093/beheco/11.1.110 [Google Scholar]
- Forster G.R. The biology of the common prawn Leander serratus Pennant. J. Mar. Biol. Assoc. UK. 1951;30:333–360. [Google Scholar]
- Kendal R.L, Coolen I, van Bergen Y, Laland K.N. Trade offs in the adaptive use of social and asocial learning. Adv. Stud. Behav. 2005;35:333–379. [Google Scholar]
- Krause J, Ruxton G.D. Oxford University Press; Oxford, UK: 2002. Living in groups. [Google Scholar]
- Laland K.N. Social learning strategies. Learn. Behav. 2004;32:4–14. doi: 10.3758/bf03196002. [DOI] [PubMed] [Google Scholar]
- Odling-Smee L, Braithwaite V.A. The role of learning in fish orientation. Fish Fish. 2003;4:235–246. [Google Scholar]
- Peres C.A. Anti-predation benefits in a mixed-species group of Amazonian tamarins. Folia Primatol. 1993;61:61–76. doi: 10.1159/000156732. [DOI] [PubMed] [Google Scholar]
- Pitcher T.J, Parrish J.K. Functions of shoaling behaviour in teleosts. In: Pitcher T.J, editor. Behaviour of teleost fishes. Chapman and Hall; London, UK: 1993. pp. 363–439. [Google Scholar]
- Ward A.J.W, Hart P.J.B, Krause J. The effects of habitat- and diet-based cues on association preferences in three-spined sticklebacks. Behav. Ecol. 2004;15:925–929. doi:10.1093/beheco/arh097 [Google Scholar]
- Ward A.J.W, Holbrook R.I, Krause J, Hart P.J.B. Social recognition in sticklebacks: the role of direct experience and habitat cues. Behav. Ecol. Sociobiol. 2005;57:575–583. doi:10.1007/s00265-004-0901-7 [Google Scholar]
- Ward A.J.W, Webster M.M, Hart P.J.B. Social recognition in wild fish populations. Proc. R. Soc. B. 2007;274:1071–1077. doi: 10.1098/rspb.2006.0231. doi:10.1098/rspb.2006.0231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster M.M, Hart P.J.B. Substrate discrimination and preference in foraging fish. Anim. Behav. 2004;68:1071–1077. doi:10.1016/j.anbehav.2004.04.003 [Google Scholar]
- Webster M.M, Hart P.J.B. Subhabitat selection by foraging threespine stickleback (Gasterosteus aculeatus): previous experience and social conformity. Behav. Ecol. Sociobiol. 2006;60:77–86. doi:10.1007/s00265-005-0143-3 [Google Scholar]
- Webster, M. M., Goldsmith, J., Ward, A. J. W. & Hart, P. J. B. In press. Habitat specific chemical cues influence association preferences and shoal cohesion in fish. Behav. Ecol. Sociobiol (doi:10.1007/s00265-007-0462-7)