Abstract
Toothed whales echolocating in the wild generate clicks with low repetition rates to locate prey but then produce rapid sequences of clicks, called buzzes, when attempting to capture prey. However, little is known about the factors that determine clicking rates or how prey type and behaviour influence echolocation-based foraging. Here we study Blainville's beaked whales foraging in deep water using a multi-sensor DTAG that records both outgoing echolocation clicks and echoes returning from mesopelagic prey. We demonstrate that the clicking rate at the beginning of buzzes is related to the distance between whale and prey, supporting the presumption that whales focus on a specific prey target during the buzz. One whale showed a bimodal relationship between target range and clicking rate producing abnormally slow buzz clicks while attempting to capture large echoic targets, probably schooling prey, with echo duration indicating a school diameter of up to 4.3 m. These targets were only found when the whale performed tight circling manoeuvres spending up to five times longer in water volumes with large targets than with small targets. The result indicates that toothed whales in the wild can adjust their echolocation behaviour and movement for capture of different prey on the basis of structural echo information.
Keywords: echolocation, beaked whale, schooling, mesopelagic
1. Introduction
Toothed whales foraging in deep water must rely partially, if not fully, on echolocation to locate and capture prey but, while echolocation has been studied intensively in captive toothed whales, little is known about its use by toothed whales in the wild (Au 1993). The recent development of archival acoustic recording tags has opened a new window for studies of echolocation in free-ranging toothed whales (e.g. Madsen et al. 2002; Johnson & Tyack 2003; Akamatsu et al. 2005). It has become apparent that toothed whale species, studied in the wild, employ an acoustic behaviour similar to that of echolocating bats (Griffin 1958; Madsen et al. 2002) involving relatively slow clicking while searching for, and approaching, prey items, and rapid sequences of clicks (coined buzzes) during the final stages of capture (Johnson et al. 2004; Miller et al. 2004).
Ironically, the most detailed information on echolocation in free-ranging toothed whales has come from one of the least known and most enigmatic cetacean families, the beaked whales. Acoustic tags on beaked whales have recorded both the outgoing echolocation clicks and the returning echoes from targets in the water column, including prey (Johnson et al. 2004). Studies on Blainville's beaked whales (Mesoplodon densirostris) off the Canary Islands have revealed that they produce frequency-modulated (FM) echolocation clicks at 0.2–0.6 s intervals to detect and approach individual prey items. When prey are some 3–4 m away, tagged whales switch to a high repetition rate buzz, comprising lower energy unmodulated clicks, during which a prey capture attempt is made (Madsen et al. 2005; Johnson et al. 2006). The inter-click intervals (ICIs) during search and approach are much longer than the two-way travel time (TWTT) to the apparent target and, in the limited data available, do not seem to vary in a manner consistent with the reducing range while approaching prey (Madsen et al. 2005). This observation, which is at odds with reports from some bats (Griffin 1958) and some trained delphinids (Au 1993), was interpreted as representing a preference for maintaining a broad acoustic scene in the cluttered environments that can be encountered by toothed whales foraging in the wild (Madsen et al. 2005). The pattern of click production during a small set of buzzes recorded from another Blainville's beaked whale also seemed to be quite stereotyped (Johnson et al. 2006), again suggesting a lack of adaptation in click rate to specific targets, a surprising outcome if buzzes represent a time in which attention is focused on a single prey. At this point, the factors determining the production rate of search and buzz clicks in the wild, effectively the sampling rate of the biosonar system, must be considered unknown and a dataset of sufficient size to test specific hypotheses has been slow to accumulate.
Here we present data from three Blainville's beaked whales tagged off the Canary Islands and the Bahamas to explore rate adjustment in buzz clicks in relation to the distance and type of prey echoes. Using high-resolution echograms to visualize prey echoes prior to, and during buzzes, we show that the rate of clicking at the start of buzzes is, in fact, related to target distance in all three whales. However, a subset of buzzes, produced by one of the whales in response to apparent large congregations of prey, deviates substantially from the general trend providing an opportunity to study how echolocation behaviour is adapted to echo characteristics. The results, albeit limited by the size of the dataset, reveal a dynamic beaked whale echolocation system and provide insight into factors that may influence biosonar use during prey capture.
2. Material and methods
Blainville's beaked whales were tagged with stereo DTAG acoustic recording tags (Johnson & Tyack 2003) off the coast of El Hierro in the Canary Islands and off Andros Island in the Bahamas (table 1). All three tags were attached with suction cups to the upper dorsal surface approximately midway between the blowhole and the dorsal fin. Sensors on the tag, including pressure and orientation transducers, were sampled at 50 Hz throughout the attachments while signals from the two hydrophones, sampled synchronously at 192 kHz per channel, were recorded for about the first half of each attachment (table 1). Acoustic data were examined in Matlab to determine the production time of each echolocation click made by the tagged whale (Johnson et al. 2006). Click sequences with ICI below 0.1 s were considered to be buzzes based on the bimodal distribution of ICI (Madsen et al. 2005), and an echogram was constructed for each buzz by stacking the envelopes of 20 ms segments of sound, synchronized to each click in the buzz. To visualize the targets present just before the buzz, FM clicks up to 10 s prior to the buzz and were also used in forming the echogram. Echograms were displayed with a time ordinate instead of a click-number ordinate by drawing each envelope as a coloured bar with thickness determined by the preceding ICI (figure 2).
Table 1.
Tag data summary and results from first-order regression of the TWTT to the furthest edge of the target against the initial ICI in buzz for three Blainville's beaked whales. (Buzzes with a preceding buzz within 6 s were eliminated (see text) and the extrema in ordinate and abscissa were trimmed before regression. All regressions had p<0.001. The residuals for Md04_287a showed some heteroscedasticity and so the confidence limits on the derived parameters may be optimistic.)
| ID | date | location | durationa | divesb | no. of buzzesc | r2 | F (d.f.) | sloped | offsetd (ms) |
|---|---|---|---|---|---|---|---|---|---|
| Md04_287a | 13 Oct 2004 | El Hierro | 9.6/15.4 | 4/9 | 133/126/91 | 0.62 | 122 (76) | 1.8 (1.5–2.1) | 2.3 (0.3–4.5) |
| Md05_285a | 12 Oct 2005 | El Hierro | 8.6/17.4 | 4/7 | 166/151/111 | 0.44 | 63 (81) | 0.8 (0.6–1.0) | 8.1 (7.1–9.2) |
| Md06_296a | 23 Oct 2006 | Andros Is. Bahamas | 10.7/19.4 | 4/7 | 243 | ||||
| ICI<0.02 s | /216/48 | 0.36 | 23 (41) | 0.6 (0.4–0.9) | 8.2 (6.6–9.9) | ||||
| ICI>0.02 s | /15/15 | 0.74 | 29 (10) | 2.2 (1.3–3.2) | 12.4 (5.2–19.5) |
Duration of audio recording/duration of sensor recording, hours.
Deep foraging dives with audio recording/deep dives with sensor recording.
Total number of buzzes recorded/buzzes processable for ICI/buzzes with apparent prey echo.
Parameter estimate and confidence limits at 0.05 level for first-order regression of ICI against TWTT. Md04_287a and Md06_296a were females while Md05_285a was indeterminate.
Figure 2.
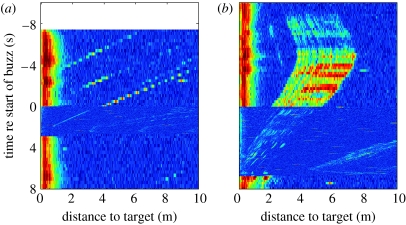
Echograms during capture attempts of (a) small and (b) large prey targets by a Blainville's beaked whale in the Bahamas. FM clicks are produced prior to and following the buzz while weaker buzz clicks are produced from time (a) 0–2.7 s and (b) 0−6.6 s. The initial buzz ICIs are (a) 0.012 s and (b) 0.029 s, while the echo extents are (a) 0.3 m and (b) 3.2 m. The closing speeds just prior to the buzzes are (a) 1.5 m s−1 (1.58 m closed in 1.05 s during the final five FM clicks) and (b) 0.6 m s−1 (0.55 m closed in 0.95 s during the final five FM clicks). The uncoloured region at the top of the left panel indicates a 4.4 s pause in clicking.
Individual targets were evident in the echograms as sequences of echoes with consistently varying TWTT evinced by consecutive clicks. A subset of buzzes contained an echo train that coincided with an echo train just prior to the buzz both in terms of distance and closing speed (figure 2; Johnson et al. 2006), and this was interpreted as a contiguous sequence of echoes from the organism targeted during the buzz. A few buzzes were found to involve the same target as that of a closely preceding buzz (i.e. the prey was not captured in the first buzz and a second capture attempt was made). To avoid this source of serial correlation, all buzzes starting within 6 s (the length of the longest interval between buzzes with an apparent shared target) of the end of a previous buzz were excluded from analysis.
For each buzz with an apparent target, the duration of the buzz and the initial ICI of the buzz clicks were measured. As click production accelerates rapidly at the start of buzzes, the mean ICI over 20 clicks near the start of the buzz (in fact, clicks 6–25 in the buzz) was taken as representing the initial ICI. This average typically covers the interval from 0.1 to 0.35 s into the buzz. In buzzes with an apparent target, the closing speed was estimated by making a linear fit between the generation time of the last three to five FM clicks before the buzz and the TWTT to a persistent feature in the target echo. The number of clicks included in the fit varied as a clear echo is not obtained for every click. The TWTT to the nearest and furthest edge of the target echo on the last FM click before the buzz was also measured. The target edges were considered to occur when the envelope of the bandpass-filtered (27–51 kHz) echo rose more than 10 dB above, or fell to within 10 dB, of the contemporaneous ambient noise level. The bandpass filter was chosen to cover the frequency band of the forward-directed FM clicks of Blainville's beaked whales (Johnson et al. 2006). Both the closing speed and target edges were computed for the FM clicks just prior to the buzz as echoes with adequate signal-to-noise ratio were more consistently evinced for the FM clicks than for the buzz clicks. The one-dimensional extent of the target was taken as the difference in the TWTT to the furthest and nearest target edges. Given the variability in aspect and insonification of targets, the echo extent may be a more robust measure of target size (or target cluster size, in the case of multiple, closely spaced organisms) than the echo level. To convert to metres, time differences were multiplied by one-half of the sound speed from representative conductivity-time–depth profiles taken at each study site.
To examine the relationship between target size and whale movement, we first estimated the swim speed using a Kalman filter matching pitch angle to depth rate, and then combined this with the tag orientation sensors to compute the dead-reckoned track of the whale (Johnson & Tyack 2003) sampled at 0.2 s intervals. Various measures of tortuosity have been proposed in the analysis of animal paths, commonly based on the ratio of straight-line distance covered versus path length over some time interval (Benhamou 2004; Wilson et al. 2007). While these statistics reveal cursive motion, they do not distinguish progressive movements from circling movements where the animal stays in the same volume. Here we use the residence time of the whale within a pre-determined volume calculated as follows: at each point on the track, the number of track samples lying within a sphere of radius r, centred on the current point, are recorded. This number is divided by the track sampling rate (5 Hz) to compute residence time in seconds and then divided by 2r to produce a residence index with dimension seconds per metre. Here we use a radius r=20 m although similar results were obtained for radii from 10 to 50 m.
3. Results
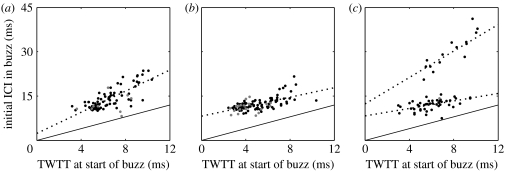
The tagged whales performed deep dives to maximum depths of 711–1251 m (Md04_287a), 657–1011 m (Md05_285a) and 817–1070 m (Md06_296a). Echolocation sounds comprising FM clicks and buzzes were produced throughout the base of each foraging dive. Dive cycle and sound production were broadly similar in the Bahamas and Canary Islands (Madsen et al. 2005; Johnson et al. 2006; Tyack et al. 2006), although the Bahamian whale produced notably more buzzes per foraging dive (table 1). In the Canarian whales, over 70% of processable buzzes (i.e. those for which most buzz clicks could be isolated) had detectable prey echoes while echoes were only detected in some 27% of the buzzes produced by the Bahamian whale. Although the ambient noise level in the two locations was comparable, the difference in target detection rate may be due to a more posterior tag position on the Bahamian whale or the targeting of generally lower target strength prey by this whale. Nonetheless, for the set of buzzes with detectable prey echoes, significant positive correlations were found for all three whales between target range (parametrized as TWTT to the furthest edge of the target) just before the start of the buzz, and the buzz click ICI adopted at the beginning of the buzz (table 1; figure 1).
Figure 1.
TWTT to the furthest edge of target versus ICI at the beginning of buzzes by three Blainville's beaked whales: (a) Md04_287a, (b) Md05_285a (Canary Islands) and (c) Md06_296a (Bahamas). The dotted lines indicate the regression while the solid lines indicate the relation ICI=TWTT, that is, the case in which the subsequent click is produced immediately after reception of the echo from the previous click. The points with ICI>20 ms in (c) seem to represent a distinct echolocation behaviour not seen in the whales tagged in the Canary Islands. Grey dots indicate buzzes that closely follow another buzz and were excluded from the regression.
For the Bahamian whale, a subset of buzzes with unusually long ICI appeared to follow a different TWTT–ICI relationship than did the remainder of the buzzes (figure 1c). Testing combined and separated regression models for this whale gave strong support (F2,58=390, p<0.001) for separating the buzzes into two ICI groups as in table 1. Given that no initial buzz ICIs between 15.5 and 20.6 ms were observed in the 231 processable buzzes, 20 ms was chosen as the separation threshold and we coin the terms ‘slow’ and ‘normal’ for buzzes with initial ICI greater and less than 20 ms, respectively. The ICI in both types of buzzes decreased continuously during the first half of the buzz (Johnson et al. 2006) but the minimum ICI attained in slow buzzes was significantly higher than for normal buzzes (rank-sum p<0.001; median minimum ICI slow buzz: 7.1 ms and normal buzz: 4.3 ms).
Comparing other attributes of buzzes for the Bahamian whale revealed that slow buzzes were significantly longer than normal buzzes (rank-sum p<0.001; median duration slow buzz: 6.2 s and normal buzz: 1.9 s). The closing speed (i.e. the range rate of the prey echo) at the start of slow buzzes was also significantly lower than for normal buzzes (rank-sum p<0.001; median closing speed slow buzz: 0.6 m s−1 and normal buzz: 1.4 m s−1). On visual inspection of the echograms for slow and normal buzzes, we found that slow buzzes were uniformly associated with distinctive large targets (figure 2b) which appeared to be composed of multiple discrete reflections or glints. In comparison, the only normal buzz with a target of this kind occurred 2 s after a slow buzz and clearly involved a portion of the same prey targeted in the preceding buzz. To quantify the target size of visually appraised echoes, we measured the extent of prey echoes finding that slow buzzes were strongly associated with large echo extent targets (rank-sum p<0.001; median echo extent slow buzz: 2.7 m and normal buzz: 0.4 m) with a maximum radial extent of 4.3 m. Although 16 of 64 prey echoes in the Bahamian recording were judged to be large targets, this target type was rare in the Canary Island recordings: no examples were found for Md05_285a while 6 of the 91 Md04_287a buzzes with detectable prey echoes were judged to involve large targets. However, three of these six buzzes appeared to target the same prey item leaving four examples that could be considered independent. The initial ICI and buzz duration for these four buzzes did not follow the same pattern as for the Bahamian whale, although it is impossible to say whether this reflects a geographical difference given the small dataset available.
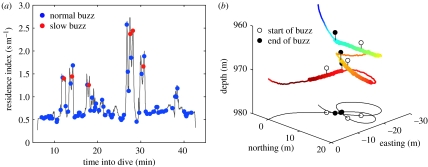
Thus, the distinctive slow buzzes produced by the Bahamian whale are associated with large targets, low closing speeds and long buzz durations. To explore other possible correlates, we examined the movement of the Bahamian whale while foraging. Slow buzzes tended to be made at slightly greater depths than normal buzzes (rank-sum p=0.01; median depth slow buzz: 978 m and normal buzz: 943 m) but a strong connection was found between slow buzzes and track tortuosity (figure 3). The residence index of track segments with slow buzzes was significantly greater than that for normal buzzes (rank-sum p<0.001; median residence index slow buzz: 1.5 and normal buzz: 0.7), and all slow buzzes occurred with a residence index greater than 1.2. The whale spent between two and five times more time within 20 m of the locations of slow buzzes than it did for most normal buzzes, achieving this by swimming in circling tracks (figures 3 and 4).
Figure 3.
Tortuosity during the deep foraging phase of a dive recorded from the Bahamian Blainville's beaked whale. (a) Residence index as a function of dive time showing occasional episodes in which the whale lingered within a small (less than 20 m radius) volume. (b) Reconstructed track segment during a tortuous episode (residence index 2.3) with colour indicating depth. The 90 s segment starts (at horizontal position 0, 0) 15 s before a buzz and covers three buzzes with prey echo extents of 0.7, 0.7 and 3.9 m. The targets of the second and third buzzes were both judged visually to be large targets but the poor signal-to-noise ratio of the second target resulted in a low computed echo extent. Wider tracks indicate where prey echoes were detected in the tag recording.
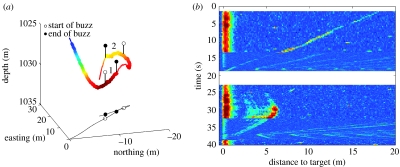
Figure 4.
Echoes observed during a 40 s tortuous track segment (residence index 1.3). (a) Track segment annotated as in figure 3. (b) Echogram observed during the track segment. A small target (echo extent 0.8 m) was observed during the approach to buzz 1 while a large compound target (echo extent 3.5 m) was observed shortly after in essentially the same location. Pauses in clicking occurred at times 0 and 20 s during the segment.
Slow buzzes tended to occur in bouts within the same tortuous track segment, as in the examples of figures 3 and 4. While the preceding inter-buzz interval (IBI, i.e. the time elapsed from the end of one buzz to the start of the next buzz) did not differ for slow and normal buzzes (rank-sum p=0.5; median IBI prior to slow buzzes: 17 s and normal buzzes: 24 s), the shortest inter-buzz distance (IBD) between the end locations of one buzz and of any preceding buzz, i.e. the shortest distance between the presumed capture locations, was significantly less for slow buzzes (rank-sum p<0.001; median shortest preceding IBD slow buzz: 4 m and normal buzz: 31 m). Although this is a natural consequence of the whales circling track while producing slow buzzes, it is noteworthy that 13 of the 15 slow buzzes ended within 10 m of the end location of a preceding normal buzz with a small prey echo as in figures 3 and 4 raising the question of why the large targets pursued in these slow buzzes were not visible in the echogram of the preceding buzz despite apparent overlap in the water volumes searched (figure 4).
4. Discussion
Several factors influence the rate of sound production by an echolocating predator. A high clicking rate will increase the opportunity to react to moving prey, but could give rise to range ambiguity if a new click is emitted before echoes from distant targets have arrived. Conversely, a low clicking rate may allow echoes from more distant targets to be included in a cognitive auditory scene at the expense of a reduced tracking capability. The trade-off between these factors is probably solved dynamically depending on the distance to potential prey, their expected behaviour, and the clutter and noise levels. For dolphins and bats performing trained echolocation tasks in relatively uncluttered environments, a clicking rate matched to the range of the target (the TWTT plus processing time model) may maximize the chance of a reward (Au 1993). In contrast, a toothed whale searching for specific prey in a target-rich environment may prefer to maintain a broad auditory scene and thus a low clicking rate until it draws close enough to a prey to warrant a high update rate locked to the selected target. This latter model may explain qualitatively the radical difference in production rates of FM search clicks and buzz clicks in beaked whales and the apparent lack of target range-dependent rate adjustment in FM clicks just prior to buzzes (Madsen et al. 2005), but more specific information on click rate selection has been lacking.
Here we provide strong evidence that the clicking rate is correlated with the target range at the beginning of buzzes in three Blainville's beaked whales from two different geographical locations. Our method depends on the correct identification of the echo sequence corresponding to the selected prey in a buzz by means of a high-resolution echogram display but, as shown in figure 2, this identification is often unambiguous. All three whales showed significant correlation between the distance to the apparent target just prior to the buzz and the ICI adopted some 0.3–0.6 s later in the buzz (figure 1). We used the TWTT to the furthest edge of the target as a proxy for distance hypothesizing that, if whales are focusing on this target, they would not make the next click until after the entire echo from the current click was received (Au 1993; Wilson & Moss 2004). For a majority of targets, the sonar cross-section is small and similar results would be obtained with other definitions of target range but the distinction is important for the large echoic targets in the Bahamian recording. A plausible conclusion is that the whales focused their echolocation effort on selected targets during buzzes, setting the ICI to match the TWTT plus a, possibly individually varying and target-dependent, processing time, although the relatively low r2 figures indicate that other factors also influence the buzz ICI.
The slow buzzes associated with large compound targets (figure 2b) in the Bahamian recording provide an example of how echo characteristics may influence both echolocation behaviour and movements during prey capture attempts. These buzzes with unusually long ICI occur exclusively during tight circling movements of the whale and are marked by low prey-closing speeds and long buzz durations. A plausible explanation for this suite of behaviours is that the whale uses structural information in the echo to adapt its acoustic and locomotor behaviour for the subsequent capture attempt. In the following we consider what the apparently large targets may be and why the whale might adjust its behaviour when encountering them.
Blainville's beaked whales have small mouth apertures and are presumed to feed by suction, lacking teeth for tearing or mastication (Heyning & Mead 1996). The large sonar cross-sections of prey reported here (up to 4.3 m) then most likely result from ensonification of schools of small targets rather than single targets, equivalent in size to the whale itself. This is supported by the lack of an obvious impact, recorded acoustically or by accelerometers on the tag, and by the visual impression that the targets, as displayed in echograms, contain multiple components (figure 2b). If this is the correct interpretation, such schools of prey may be valuable targets offering the opportunity to reduce the time and oxygen spent in locating a new prey target for the next buzz. The circling behaviour of the whale coincident with the detection of these targets and the resulting longer residence time in volumes containing large targets supports the notion that the targets represent a valuable and patchy prey resource. Oddly, however, large targets were not detected in the tag recording until the whale had passed close by, and turned back towards, the location at which the target would ultimately be intercepted (figure 4). In 13 of the 15 slow buzzes with large targets, the buzz was preceded by a normal buzz in almost the same location but with a small apparent target size. Although the data size is too small to justify much speculation, one possible explanation is that the circling track of the whale prior to the slow buzz provoked a schooling behaviour, hence the large targets were not seen in preceding buzzes because they had not yet formed. A number of shallower-diving whale species have been observed to provoke schooling of prey both as an individual foraging behaviour (Nøttestad et al. 2002) and in cooperative foraging (Similä & Ugarte 1993; Benoit-Bird & Au 2003). Little is known about the deep-sea squid and fishes that beaked whales prey on (MacLeod et al. 2006; Santos et al. 2007) but some mesopelagic fauna are known to school as an anti-predator response (Kaartvedt et al. 1998) and the circling movements of the Blainville's beaked whale reported here are broadly similar to manoeuvres used by other cetaceans to school prey.
The other parameters that distinguish slow buzzes do not offer much additional insight into the possible prey targeted. The low approach speed at the beginning of slow buzzes may simply be a consequence of momentum loss during tight turning. Likewise, the long duration of buzzes associated with large targets may follow from the reduced speed at which these targets are approached. The slow initial clicking rate in long buzzes is more difficult to explain. The ICIs at the beginning of slow buzzes are three to five times longer than needed to ensure that the entire echo from large targets is received without interference (figure 1c) so the increase in target size does not seem to explain the slow clicking. The correlation between slow clicking and long buzzes suggests an effort to limit the total number of clicks produced and, indeed, the number of clicks that can be made in a buzz must be limited ultimately by the volume of air available for pneumatic sound production (Cranford et al. 1996). However, the longest buzz made by the Bahamian whale contained some 720 clicks while beaked whales tagged in the Canary Islands have produced occasional buzzes with up to three times this number of clicks. A more plausible explanation for the long ICIs is a need for increased time between clicks when processing the unusually long and complex echoes (figure 2b). It is also possible that the 20–40 ms initial ICIs in slow buzzes serve to maintain a larger auditory scene while manoeuvring around the complex targets and, in fact, the corresponding maximum echolocation ranges of 15–30 m are broadly comparable with the diameters of the circling manoeuvres associated with slow buzzes (figure 3).
Whatever the reason for the long ICIs, the combination of circling tracks, slow approaches and long and slow buzzes seems to represent a distinct biosonar tactic related to the acquisition of prey that produce distinctive long-duration echoes, logically, a schooling prey. Although we cannot speak to the prevalence of this foraging style in Blainville's beaked whales, our data demonstrate that deep-diving toothed whales can classify prey by echolocation and adapt their sonar behaviour and movements accordingly during prey capture attempts. This result, combined with the correlation between prey distance and buzz click rate, support the interpretation that echolocation in buzzes is used to acquire selected nearby prey in Blainville's beaked whales and that the sonar sampling rate in this phase of echolocation can be modified to fit the circumstances in each prey capture.
Acknowledgments
We thank D. Claridge, C. Dunn, A. Bocconcelli, O. Patterson, D. Moretti, N. DiMarzio, R. Morrissey, S. Jarvis and J. Ward for their field support in the Bahamas, and the Marine Mammal Group at the University of La Laguna (ULL) for their support in the Canary Islands. This project was funded by NOPP and SERDP. We also thank A. Brito, D. Claridge, E. Terray, P. Tyack, M. Wahlberg and the anonymous reviewers for their helpful comments. P.T.M. was funded by a Steno scholarship from FNU. Research was conducted under permit no. 1 awarded to D. Claridge by the Bahamas Government and under a permit awarded to ULL from the Canary Islands Government. Field protocols were approved by the WHOI Institutional Animal Care and Use Committee.
References
- Akamatsu T, Wang D, Wang K, Naito Y. Biosonar behaviour of free-ranging porpoises. Proc. R. Soc. B. 2005;272:797–801. doi: 10.1098/rspb.2004.3024. doi:10.1098/rspb.2004.3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au W.W.L. Springer; New York, NY: 1993. The sonar of dolphins. [Google Scholar]
- Benhamou S. How to reliably estimate the tortuosity of an animal's path: straightness, sinuousity, or fractal dimension? J. Theor. Biol. 2004;229:209–220. doi: 10.1016/j.jtbi.2004.03.016. doi:10.1016/j.jtbi.2004.03.016 [DOI] [PubMed] [Google Scholar]
- Benoit-Bird K.J, Au W.W.L. Prey dynamics affect foraging by a pelagic predator (Stenella longirostris) over a range of spatial and temporal scales. Behav. Ecol. Sociobiol. 2003;53:364–373. [Google Scholar]
- Cranford T.W, Amundin M, Norris K.S. Functional morphology and homology in the odontocete nasal complex: implications for sound generation. J. Morphol. 1996;228:223–285. doi: 10.1002/(SICI)1097-4687(199606)228:3<223::AID-JMOR1>3.0.CO;2-3. doi:10.1002/(SICI)1097-4687(199606)228:3<223::AID-JMOR1>3.0.CO;2-3 [DOI] [PubMed] [Google Scholar]
- Griffin D.R. Cornell University Press; New York, NY: 1958. Listening in the dark: the acoustic orientation of bats and men. [Google Scholar]
- Heyning J.E, Mead J.G. Suction feeding in beaked whales: morphological and observational evidence. Smithson. Contrib. Sci. 1996;464:1–12. [Google Scholar]
- Johnson M, Tyack P.L. A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J. Oceanic Eng. 2003;28:3–12. doi:10.1109/JOE.2002.808212 [Google Scholar]
- Johnson M, Madsen P.T, Aguilar Soto N, Zimmer W.M.X, Tyack P.L. Beaked whales echolocate for prey. Proc. R. Soc. B. 2004;271:S383–S386. doi: 10.1098/rsbl.2004.0208. doi:10.1098/rsbl.2004.0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Madsen P.T, Zimmer W.M.X, Aguilar Soto N, Tyack P.L. Foraging Blainville's beaked whales (Mesoplodon densirostris) produce distinct click types matched to different phases of echolocation. J. Exp. Biol. 2006;209:5038–5050. doi: 10.1242/jeb.02596. doi:10.1242/jeb.02596 [DOI] [PubMed] [Google Scholar]
- Kaartvedt S, Knutsen T, Holst J.C. Schooling of the vertically migrating mesopelagic fish Maurolicus muelleri in light summer nights. Mar. Ecol. Prog. Ser. 1998;326:295–307. [Google Scholar]
- MacLeod C.D, Santos M.B, Lópes A, Pierce G.J. Relative prey size consumption in toothed whales: implications for prey selection and level of specialisation. Mar. Ecol. Prog. Ser. 2006;326:295–307. doi:10.3354/meps326295 [Google Scholar]
- Madsen P.T, Payne R, Kristiansen N.U, Wahlberg M, Kerr I, Moehl B. Sperm whale sound production studied with ultrasound time/depth-recording tags. J. Exp. Biol. 2002;205:1899–1906. doi: 10.1242/jeb.205.13.1899. [DOI] [PubMed] [Google Scholar]
- Madsen P.T, Johnson M, Aguilar Soto N, Zimmer W.M.X, Tyack P.L. Biosonar performance of foraging beaked whales (Mesoplodon densirostris) J. Exp. Biol. 2005;208:181–194. doi: 10.1242/jeb.01327. doi:10.1242/jeb.01327 [DOI] [PubMed] [Google Scholar]
- Miller P.J.O, Johnson M, Tyack P.L. Sperm whale behaviour indicates the use of echolocation click buzzes ‘creaks’ in prey capture. Proc. R. Soc. B. 2004;271:2239–2247. doi: 10.1098/rspb.2004.2863. doi:10.1098/rspb.2004.2863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøttestad L, Ferno A, Mackinson S, Pitcher T, Misund O.A. How whales influence herring school dynamics in a cold-front area of the Norwegian Sea. ICES J. Mar. Sci. 2002;59:393–400. doi:10.1006/jmsc.2001.1172 [Google Scholar]
- Santos M.B, Martín V, Arbelo M, Fernández A, Pierce G.J. Insights into the diet of beaked whales from the atypical mass stranding in Canary Islands in September 2002. J. Mar. Biol. Assoc. UK. 2007;87:243–251. doi:10.1017/S0025315407054380 [Google Scholar]
- Similä T, Ugarte F. Surface and underwater observation of cooperatively feeding killer whales in Northern Norway. Can. J. Zool. 1993;71:1494–1499. [Google Scholar]
- Tyack P.L, Johnson M, Aguilar Soto N, Sturlese A, Madsen P.T. Extreme diving of beaked whales. J. Exp. Biol. 2006;209:4238–4253. doi: 10.1242/jeb.02505. doi:10.1242/jeb.02505 [DOI] [PubMed] [Google Scholar]
- Wilson W.W, Moss C.F. Sensory-motor behavior of free-flying FM bats during target capture. In: Thomas J, Moss C.F, Vater M, editors. Advances in the study of echolocation in bats and dolphins. University of Chicago Press; Chicago, IL: 2004. pp. 22–27. [Google Scholar]
- Wilson R.P, et al. All at sea with animal tracks; methodological and analytical solutions for the resolution of movement. Deep Sea Res. II. 2007;54:193–210. doi:10.1016/j.dsr2.2006.11.017 [Google Scholar]