Abstract
Emerging zoonotic pathogens are a constant threat to human health throughout the world. Control strategies to protect public health regularly fail, due in part to the tendency to focus on a single host species assumed to be the primary reservoir for a pathogen. Here, we present evidence that a diverse set of species can play an important role in determining disease risk to humans using Lyme disease as a model. Host-targeted public health strategies to control the Lyme disease epidemic in North America have focused on interrupting Borrelia burgdorferi sensu stricto (ss) transmission between blacklegged ticks and the putative dominant reservoir species, white-footed mice. However, B. burgdorferi ss infects more than a dozen vertebrate species, any of which could transmit the pathogen to feeding ticks and increase the density of infected ticks and Lyme disease risk. Using genetic and ecological data, we demonstrate that mice are neither the primary host for ticks nor the primary reservoir for B. burgdorferi ss, feeding 10% of all ticks and 25% of B. burgdorferi-infected ticks. Inconspicuous shrews feed 35% of all ticks and 55% of infected ticks. Because several important host species influence Lyme disease risk, interventions directed at a multiple host species will be required to control this epidemic.
Keywords: Borrelia burgdorferi, ospC, primary reservoir, zoonotic disease
1. Introduction
Pathogenic microbes are emerging and re-emerging at an alarming rate. Of the 175 emerging infectious diseases of humans, 132 are zoonotic (Taylor et al. 2001; Woolhouse & Gowtage-Sequeria 2005), residing in wildlife reservoir species and occasionally transmitted to humans either directly or via an intermediate vector. Zoonotic pathogens, by definition, are not specialists on any single species; they can infect humans and at least one non-human animal. A robust understanding of the transmission cycle in nature that governs the distribution and abundance of pathogens, and thus contact with humans, is essential for the effective control of emerging infectious diseases. Here, we investigate the degree to which the zoonotic pathogen that causes Lyme disease, Borrelia burgdorferi sensu stricto, is maintained and amplified by multiple reservoir host species.
Lyme disease is the most prevalent arthropod-transmitted zoonotic disease in North America due to the prevalence of B. burgdorferi-infected Ixodes scapularis ticks (Johns et al. 2001; CDC 2004). It is commonly held that the white-footed mouse, Peromyscus leucopus (hereafter ‘mouse’), is the principal wildlife host for B. burgdorferi ss and for immature stages of the tick vector in the eastern and central endemic zones (Schwan et al. 1989; Mather & Mather 1990; Rand et al. 1993; Porco 1999; Ostfeld et al. 2001; Derdakova et al. 2004; Anderson & Norris 2006). Thus, it is assumed that mice are primarily responsible for the propagation and prevalence of B. burgdorferi-infected ticks and thus Lyme disease risk. Many interventions and control strategies have focused on interrupting the transmission of B. burgdorferi ss from mice to ticks in order to reduce human exposure (Deblinger & Rimmer 1991; Dolan et al. 2004; Tsao et al. 2004; Hornbostel et al. 2005). These interventions have resulted in only modest success in reducing B. burgdorferi ss infection prevalence in I. scapularis and thus minimal reductions in human Lyme disease risk. Although the modest success may be due to methodology, it is feasible that the pervasive, yet often implicit, assumption that mice dominate the host-to-tick transmission cycle is incorrect (Mather et al. 1989; Schmidt & Ostfeld 2001).
One possible explanation for the weak effects of mouse-based interventions is the existence of important alternative host species that can maintain an effective host-to-tick transmission cycle in addition to mice. Their ability to do so depends on both species-specific reservoir competence (the probability that an infected host transmits B. burgdorferi ss to feeding ticks) and the proportion of the tick population that feeds on that host species. Mather et al. (1989) coined the term ‘reservoir potential’ to represent the proportion of the total population of infected ticks contributed by each host species. White-footed mice are heavily parasitized by immature (larval and nymphal) ticks and more than 85% of mouse-fed ticks acquire B. burgdorferi ss (Mather et al. 1989; LoGiudice et al. 2003; Brisson & Dykhuizen 2004). These observations suggest that mice have high reservoir potential and have contributed to the assertion that mice are the primary host for both immature ticks and B. burgdorferi ss. Host species other than mice have significantly lower reservoir competences (Telford et al. 1990; Markowski et al. 1998; Richter et al. 2000; LoGiudice et al. 2003; Brisson & Dykhuizen 2004; Hanincova et al. 2006). Species with lower reservoir competence may have stronger reservoir potential than mice if they supply blood meals to a substantially greater proportion of immature ticks, either through greater densities or behaviours that increase the tick burden per individual.
In this article we use the genotypic variability within B. burgdorferi ss populations to determine the reservoir potential of host species (Wang et al. 1999; Qiu et al. 2002). Fifteen serotypically distinct genotypes, identified by the allele at the outer surface protein C (ospC) locus, coexist as a stable polymorphism in typical B. burgdorferi ss populations in the northeastern USA (Qiu et al. 2002; Brisson & Dykhuizen 2004; Earnhart et al. 2005). Previous data suggest that each vertebrate species transmits only a subset of these B. burgdorferi ss genotypes to feeding larvae at a consequential frequency (Brisson & Dykhuizen 2004; Hanincova et al. 2006). As larval ticks hatch free of B. burgdorferi ss, the genotypes in a nymph are always acquired from the larval blood meal host (Magnarelli et al. 1987). Thus, the combination of genotypes in a nymphal tick implicates a vertebrate species as the source of that larval blood meal. In this report, we use B. burgdorferi ss genotype data, along with other molecular and ecological data, to estimate the distribution of larval tick meals on vertebrate host species as well as the proportion of the infected tick population that acquire B. burgdorferi ss from each species. These results reflect the reservoir potential of each species and the ability of each species to amplify the prevalence and abundance of infected ticks and thus Lyme disease risk.
2. Material and methods
(a) Empirical data
Four classes of empirical data were used in this study: (i) the ospC genotypes in host-seeking nymphs, (ii) the transmission probabilities of each ospC genotype from five vertebrate host species to feeding larval ticks, (iii) the number of larval I. scapularis ticks feeding on individuals of each of 11 vertebrate species, and (iv) the population densities of each of these vertebrate species.
The ospC genotypes in 188 host-seeking nymphs were determined. Nymphs were collected in 2002 in an oak/maple forest at the Institute of Ecosystem Studies (IES) in Dutchess County, NY (Brisson & Dykhuizen 2004).
The transmission probabilities—the proportions of larvae feeding on a species that become infected with each genotype—from P. leucopus (white-footed mouse), Tamias striatus (chipmunk), Blarina brevicauda (short-tailed shrew) and Sciurus carolinensis (grey squirrel) were determined from animals collected in 2002 from oak/maple forests at the IES, and reported in Brisson & Dykhuizen (2004). The transmission probabilities for Sorex cinereus, the masked shrew, were determined from 119 fully engorged larvae that had fed on six individuals captured at IES using PCR–RLB (Brisson & Dykhuizen 2004). Transmission probabilities differ (a) among B. burgdorferi ss genotypes within a host species and (b) among host species for each B. burgdorferi ss genotype, but not among individuals within a host species for all host species. The host-species specific transmission probabilities of each B. burgdorferi ss genotype were calculated from the total larvae examined from each host species.
An estimate of the number of larvae that feed on individuals of each of 11 species has been previously reported (LoGiudice et al. 2003). We confirmed these estimates for mice, chipmunks, short-tailed shrews and squirrels collected at IES in 2002–2005 (data not shown).
Population densities of mice, chipmunks and deer were estimated at IES using the Jolly-Seber model with mark-recapture data input for the rodents (Arnason & Schwarz 1999), and as minimum number alive using deer sightings (Ostfeld et al. 2006a). Population densities for the remaining species were enumerated as the median of value from literature estimates (Fitch & Sandidge 1953; Sanderson 1961; Holmes & Sanderson 1965; Mosby 1969; Richens 1974; Montgomery et al. 1975; Hoffmann & Gottschang 1977; Thompson 1978; Wade-Smith & Verts 1982; Merritt 1987; Getz 1989; Wilson & Reeder 1993; Riley et al. 1998; Whitaker & Hamilton 1998; Wilson & Ruff 1999; Getz et al. 2004). Published data for this purpose were taken only from studies in nearby geographical regions and with similar forest species compositions.
(b) Analyses
We used two independent methodologies to estimate the proportions of infected host-seeking nymphs that acquired their infection from each vertebrate host species as well as the proportion of all nymphs that fed on each species. Both methodologies use the empirically determined transmission probabilities of each B. burgdorferi ss genotype from each vertebrate species to feeding ticks and the genotypes found in host-seeking nymphs. The first method, called ‘signature matching’, identifies the host species an infected nymphal tick fed upon as a larva based upon the combination of B. burgdorferi ss genotypes harboured in that tick and the genotypes regularly transmitted by each host species. The second method is an extension of the inverse model presented in Brisson & Dykhuizen (2006). These methods differ in that signature matching compares the combination of genotypes in each host-seeking nymph with the combinations that could be transmitted from each animal species to assign each tick to a particular host species. The inverse modelling approach assesses the combination of host species, given their unique genotype transmission probabilities, that could result in the ospC frequency distribution discovered in the host-seeking nymph population.
(c) Signature matching
Each vertebrate species regularly transmits a different set of B. burgdorferi ss genotypes (Brisson & Dykhuizen 2004; Hanincova et al. 2006), what we are deeming as the ‘signature’ of that species. Ticks are classified as having fed on a species if they harbour a subset of the genotypes transmitted by the vertebrate species in question; they ‘matched’ the signature. Ticks that match the signature of more than one species were excluded in this analysis. In a second analysis, ticks matching two species were assigned to each species as a half a tick; ticks that match three or more species were never assigned. The total number of the 188 ticks examined (infected and uninfected) fed by each species can be calculated as the number of ticks infected by a species divided by the species reservoir competence (LoGiudice et al. 2003; Brisson & Dykhuizen 2004).
In a second signature matching methodology, the probability with which an infected tick took its larval blood meal from a host species was calculated as the product of the empirically determined transmission probabilities from that species for each genotype found in the tick. Ticks were assigned to this species if the probability the tick fed on this species was more than twice the probability it fed on any other species. If the probability with which a tick came from a species was not twice that of any other species, the tick was excluded in one analysis or each of the two most probable species was assigned half of that tick. Ticks were excluded from all analyses if (i) more than two species had approximately equal probability (less than twofold different) of feeding a tick or (ii) the probability a tick fed on the most probable species was below 1/75. This cut-off was chosen because the transmission probabilities were usually calculated from 75 infected larvae from each host species.
(d) Inverse modelling approach
The inverse model is an extension of Brisson & Dykhuizen (2006) that calculates the proportion of host-seeking nymphs infected with genotype i (Fi) from the proportion of ticks that feed on species j (Pj) times the transmission probability of genotype i from species j (Tij) summed across all species. There are 15 linear equations, one for each ospC genotype
| (2.1) |
Fi represents the frequency of genotype i in the host-seeking nymphal population including uninfected ticks, not from any particular animal or species. The frequencies of genotypes in nymphs are a consequence of the distribution of larval blood meals taken from all host species. Pj describes the proportion of ticks that survived to the nymphal stage as a result of a blood meal taken from species j (Lord 1993). The proportion of infected ticks that took larval blood meals from each host species can be calculated from estimates of Pj as the percentage of nymphs that acquired B. burgdorferi ss from species j divided by the proportion of nymphs that are infected. The former value was calculated as the product of Pj and the proportion of ticks infected after feeding on species j; the latter value observed in host-seeking nymphs collected at IES. This model assumes that genotypes have independent transmission dynamics and no population level epistasis.
(e) Inverse model simulation
This is called an inverse model because the unknown Pj are independent variables while the dependent variable Fi is known and the equations cannot be rearranged to make Pj the dependent variable. Pj values are estimated as the combination of Pj resulting in the distribution of Fi that best fit the measured genotype frequencies in the host-seeking nymph population (Fi(measured)). Each of 1010 permutations of Pj resulted in a goodness-of-fit score (G), calculated as sum of the deviations of Fi(simulated) from Fi(measured) across all genotypes.
The B. burgdorferi ss genotype transmission probability distribution cannot be distinguished for species with very low reservoir competence (LRC) (squirrels, raccoons, deer, opossums, skunks, etc. (LoGiudice et al. 2003; Hanincova et al. 2006)). Therefore, the minimum reservoir competence necessary to produce a distinguishable transmission probability distribution was determined. First, species that infect less than 20% of the ticks that feed on them were combined into one conglomerate group, the LRC group. The model estimated the transmission probabilities of B. burgdorferi ss genotypes from the LRC group to feeding ticks while simultaneously estimating the proportion of nymphs that took their larval blood meal from five ‘species’. These species include the LRC group as well as the four species that infect a large proportion of the ticks that feed on them—white-footed mice (85%), eastern chipmunks (55%), short-tailed shrews (42%; Brisson & Dykhuizen 2004) and masked shrews (49.6–57.3%). The transmission probabilities of the LRC group as estimated by the model was compared with the genotype transmission probabilities empirically determined for grey squirrels because squirrels have the highest reservoir competence of the species represented in the LRC group (14–19%). The transmission probabilities of the LRC group were then restricted to match that of squirrels to assess the effect of assuming the transmission probability of squirrels represents every LRC species on the estimates of the distribution of larval blood meals among the vertebrate community.
(f) Sensitivity analysis
This model explicitly includes only five vertebrate species, although several other vertebrate species are parasitized by I. scapularis that may substantially affect the frequency of B. burgdorferi ss genotypes (Anderson 1988). Thus, the accuracy of the estimates of Pj may be sensitive to the inclusion of species in LRC as opposed to including each separately. To examine the potential for this bias, we removed each of the species included in the original model, forcing them into LRC, such that the model contained the other species plus the LRC category to determine how the parameter estimates are affected when species are excluded.
We tested the sensitivity of this model to empirical errors in transmission probability estimation by increasing: (i) individual transmission probabilities in a focal species by 20%, (ii) all transmission probabilities in a focal species by 20%, and (iii) all transmission probabilities in all species by 20%. Twenty per cent was chosen as it is more than twice the measured variance in transmission probability for any genotype among individuals of any species.
(g) Model comparison
Results from the signature matching and inverse modelling analyses were compared with estimates derived from conventional live-trapping methods to determine animal densities (LoGiudice et al. 2003). The proportion of larvae that feed on a vertebrate species can be calculated as the product of the vertebrate density (Dj) and the larval burden (Bj) divided by the total number of larvae that fed on any species
| (2.2) |
3. Results
(a) Masked shrews
Seventeen masked shrews were caught in pitfall traps in the late summer at IES. Six were found dead in traps, three perished within 24 h, one died before the end of the second night and seven were released. More than 20 fully engorged larvae were collected from four shrews, 12 and 9 ticks were collected from two others and fewer than five fully engorged larvae were collected from the remaining five. We determined the genotypes in the ticks collected from the six shrews from which more than nine engorged larvae were collected; the transmission probabilities for genotypes did not differ among individuals (hierarchical log-linear analysis of frequencies, p>0.05 (Sokal & Rohlf 1995)). The genotype transmission probabilities of Sorex cinereus were calculated from 66 infected ticks of the 119 ticks recovered (table S1). The percentage of larval ticks that became infected after their blood meal from a masked shrew was 55.4%, similar to that found previously (LoGiudice et al. 2003). S. cinereus commonly transmit genotypes A, B, D, F, H, K and M.
Table 1.
The reservoir potential of vertebrate species. (Column 1, signature matching; column 2, signature matching allowing one low transmission genotype; column 3, signature matching allowing one half of each tick that matched two species to be assigned to both species; column 4, signature probability matching; column 5, signature probability matching allowing one half of each tick that matched two species to be assigned to both species; column 6, estimate of the percentage of infected ticks fed by each species from an inverse model that explicitly includes mice, chipmunks and two shrew species; column 7, estimate of the percentage of infected ticks fed by each species from a model that explicitly includes mice, chipmunks, two shrew species and squirrels. Squirrels were indistinguishable from the other incompetent reservoir species using this model framework.)
| species | reservoir competence (%) | 1 (%) | 2 (%) | 3 (%) | 4 (%) | 5 (%) | 6 (%) | 7 (%) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| mouse | >85a,b | 24.4 | 26.7 | 28.3 | 23.5 | 25.0 | 25.4 | 25.8 | ||
| chipmunk | 55a,b | 4.4 | 8.9 | 10.4 | 5.9 | 8.3 | 10.5 | 12.9 | ||
| ST shrew | 42a,b | 20.0 | 20.0 | 20.8 | 27.4 | 25.0 | 25.9 | 23.9 | ||
| masked shrew | 49.6–57.3c | 26.7 | 31.1 | 26.4 | 25.5 | 26.7 | 28.9 | 27.3 | ||
| squirrel | 15–19a,b | 0 | 0 | 2.8 | 0 | 3.3 | ||||
| birds (several species) | 11.7b | |||||||||
| striped skunk | 9.7b | 24.5 | 13.3 | 11.3 | 17.7 | 11.7 | 9.2 | 10.1 | ||
| deer | 4.6b | |||||||||
| opossum | 2.6b | |||||||||
| raccoon | 1.3b | |||||||||
(b) Signature matching
Signature matching is designed to assess the vertebrate species from which an infected nymph received its larval blood meal by comparing the ospC genotypes in the tick with the set of genotypes regularly transmitted by each host to feeding larvae. Of the 66 infected nymphs, six could not be assigned to any species and 13 could be assigned to three or more species. Of the remaining 47, 34 could be definitively classified as having fed on one species given the combination of genotypes it harboured (table 1, column 1). Five additional ticks could be assigned to one species if one genotype that is rarely transmitted from a species is included in the host species signature (table 1, column 2). Assigning half a tick to two species that both regularly transmit the set of genotypes in the tick resulted in 47 of the 66 ticks assigned (table 1, column 3). This analysis suggests that at least 71.2% of infected nymphs received their larval blood meal from one of the five species with reservoir competence greater than 15%. These are probably underestimates as 13 of the 66 nymphs remained unclassified but could be assigned to three or more of these species. All three analyses suggest that at least 23.5% of infected nymphs had taken their larval meal from a mouse, 4.4% from a chipmunk, 20% from a short-tailed shrew, 25.5% from a masked shrew and less than 3% from a squirrel (table 1, columns 1–3).
The probability that a tick fed on each species can be calculated by multiplying the transmission probabilities of all genotypes present in the tick for each vertebrate species. Ticks were assigned to a species if the probability a tick fed on that species was at least twice the probability it fed on any other species (table 1, column 4). If the probability a tick fed on either of two species was similar, each species was assigned half of the tick (table 1, column 5). Nine ticks were not assigned as the probability they fed on any of these five species was less than 1 in 75 (the number of infected ticks examined for most species). Six additional ticks were not classified as feeding on a particular host species because each could be assigned to three or more species. The estimated proportion of infected ticks that fed on mice (18–20%), chipmunks (5–7%), short-tailed shrews (20–21%), masked shrews (20–24%) and squirrels (less than 3%) in the analyses described here are similar to the signature matching analyses described above.
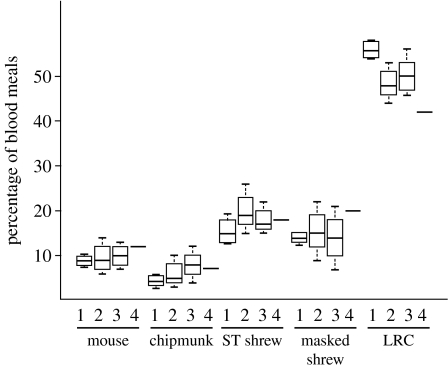
The proportion of infected and uninfected ticks fed by each species is calculated as the quotient of the number of ticks infected by a species over the reservoir competence and the total number of ticks examined. This value was similar for all five signature matching analyses performed (figure 1).
Figure 1.
Distribution of larval blood meals across host species. Estimate of the blood meals taken from each species using molecular data (box and whiskers) matches the estimate using independent ecological data (line). More than 50% of ticks take their larval blood meal from species with low reservoir competence (LRC). 1, estimates from signature matching analyses; 2, estimates from inverse model including category LRC; 3, estimates from inverse model including squirrels; 4, empirical estimates from traditional trapping methods.
(c) Inverse modelling approach
An extension of the inverse model presented in Brisson & Dykhuizen (2006) using the empirically determined transmission probabilities and the frequency distribution of genotypes in host-seeking nymphs (Fi(measured)) was employed to estimate the proportion of larval blood meals supplied by each vertebrate host species (Pj). The estimates of Pj are nearly identical for combinations with goodness-of-fit scores less than 0.025 , indicating that one area of the variable space, not several disparate but equally probable combinations, results in the best fit to the empirical data (figure S1 in the electronic supplementary material). The variance in each Pj increases slowly as combinations with greater G scores (worse fit) are added to the best-fitting combinations up to G=0.025. When variance is calculated from combinations with G>0.025, the variance for all Pj increases markedly. Here we report the average Pj from the models with goodness-of-fit scores less than 0.025 as our estimates for Pj, as opposed to the values from the single best-fitting combination, although these values are nearly identical.
The estimates from the inverse model simulation suggest that the white-footed mouse, P. leucopus, provides blood meals to approximately 10% of all larvae that successfully feed and moult to nymphs (figure 1). The eastern chipmunk, T. striatus, feeds approximately 6% of all larvae. Short-tailed shrews, B. brevicauda, and masked shrews, S. cinereus, together provide 34.5–37% of the blood meals for I. scapularis larvae, substantially more than mice and chipmunks combined. Yet, almost half (49%) of all larval ticks that successfully moult into nymphs appear to parasitize species other than mice, chipmunks or shrews.
The reservoir potential of each species can be calculated as the product of the estimates of the total nymphal population fed by each host species and the host reservoir competence divided by the proportion of nymphs that are infected (35%). Although only half of the larvae feed on mice, chipmunks and shrews combined, this analysis suggests that more than 90% of the infected nymphs acquire B. burgdorferi ss from one of these species (table 1, column 6). All other species collectively feed less than 10% of larval ticks that become infected with B. burgdorferi ss. These data indicate that 25.4 and 10.5% of all infected nymphal ticks acquired B. burgdorferi ss from mice and chipmunks, respectively, while short-tailed shrews infect 25.9% and both shrews together feed over half the infected larvae.
Including species with reservoir competence below 20% in the model does not account for a significantly higher percentage of the infected nymphal population than including only species with reservoir competence greater than 20%. The estimates of the distribution of larval blood meals across species from the models with and without squirrels (S. carolinensis: reservoir competence 14–19%) explicitly included were nearly identical (figure 1) and approximately 90% of the infected ticks still acquire B. burgdorferi ss from either a mouse, a chipmunk or a shrew (table 1, column 7). The transmission probability distributions for the LRC group of species estimated by the model were less than 10% except the estimate for genotype M from LRC (12.3%; table S1), making the distribution statistically indistinguishable from the empirically determined transmission probability distribution for squirrels (ANOVA, p>0.5, figure S2 and table S1 in the electronic supplementary material). Species in the LRC group, including squirrels, feed a substantial proportion of larvae but infect few of them with the Lyme disease spirochete.
(d) Sensitivity analysis
(i) Excluding species
We estimated Pj from simulations when each of the four species with high reservoir competence was removed from the analysis individually to determine the effect of excluding important disease hosts on the estimates of Pj (table 2). The estimates for Pj varied only slightly, with the estimate of Pj for each species explicitly included in the model increasing slightly and the LRC group increasing considerably. The estimated transmission probabilities of the LRC group also increased, generally resembling the distribution of transmission probabilities empirically estimated for the excluded species. The estimates of Pj are robust to this perturbation suggesting that no important host species are missing.
Table 2.
The estimates of Pj when individual species are excluded.
| Pmouse (%) | Pchipmunk (%) | PSTshrew (%) | Pmaskedshrew (%) | PLRC (%) | |
|---|---|---|---|---|---|
| model estimates | 11.4 | 6.3 | 18.3 | 16.5 | 47.5 |
| without mice | 0 | 9.2 | 17.3 | 23.4 | 50.1 |
| without chipmunks | 13.3 | 0 | 17.8 | 19.3 | 49.6 |
| without ST shrews | 13.2 | 7.8 | 0 | 20.7 | 58.3 |
| without masked shrews | 10.2 | 5.7 | 22.2 | 0 | 61.9 |
(ii) Transmission probabilities
The sensitivity of the model to errors in the transmission probability parameters was evaluated in three ways: by estimating Pj when (i) one transmission probability from a focal species was increased by 20%, (ii) all transmission probabilities from a focal species were increased by 20%, and (iii) all transmission probabilities from all species were increased by 20%. In the first test, Pfocalspecies decreased (table 3, A) while Pj from all other species did not change (table 3, B). When all transmission probabilities from a focal species were increased, Pfocalspecies decreased to a slightly greater extent than when individual transmission probabilities were increased (data not shown). Simultaneously increasing all transmission probabilities also depressed Pj for all species (table 3, C). The estimates of Pj appear remarkably robust to potential measurement errors in transmission probability.
Table 3.
Sensitivity to perturbations in transmission probabilities. (A, the average value of Pfocalspecies when individual transmission probabilities of the focal species are raised by 20%; B, average of Pnon-focalspecies across all simulations when transmission probabilities were increased in focal species by 20%; the difference between Pj in the original simulation and Pnon-focalspecies was nearly identical for all species; C, all transmission probabilities in all species raised simultaneously by 20%.)
| Pmouse | Pchipmunk | PSTshrew | Pmaskedshrew | PLRC | |
|---|---|---|---|---|---|
| A | 9.3%±0.03 | 4.54%±0.05 | 15.8%±0.03 | 14.35%±0.12 | X |
| B | 10.9%±0.27 | 5.63%±0.63 | 18.35%±0.38 | 17.86%±0.19 | 49.37%±0.58 |
| C | 9.0%±0.09 | 4.35%±0.08 | 15.03%±0.31 | 14.05%±0.16 | 57.57%±0.09 |
(e) Model validation
The distribution of larval blood meals and the reservoir potential estimated by signature matching are nearly equivalent to the values estimated from inverse modelling simulation. Additionally, these estimates are very similar to estimates using traditional ecological methods (figure 1).
4. Discussion
Contrary to pervasive thinking in Lyme disease ecology, the white-footed mouse, P. leucopus, is not the ‘primary’ host for either the tick vector or for the Lyme disease bacterium, B. burgdorferi ss. Although mice are common and conspicuous in habitats throughout the northeastern and midwestern USA, results from molecular and ecological data suggest that mice provide blood meals to only approximately 10% of all larvae at a representative northeastern field site (figure 1, table 2). Of these mouse-fed larvae, approximately 85% become infected, resulting in the infection of approximately 8.5% of all fed larvae (table 1). Given that on average 35% of host-seeking nymphs are infected at our field sites, we conclude that mice infect approximately 25% of the infected nymphal ticks; a substantial proportion but not enough to classify mice as the primary or principal reservoir species for B. burgdorferi ss.
Our analyses of the molecular and ecological data indicate that the realized environment of B. burgdorferi ss consists largely of four vertebrate species; 80–90% of all infected nymphs at our sites took their larval blood meal from a mouse, a chipmunk, a short-tailed shrew or a masked shrew (table 1). The signature matching analyses estimate that these species feed a slightly lower proportion of the infected nymphal population than does the inverse modelling approach (75–88% versus 89–91%, table 1). The majority of the discrepancy is caused by a depressed estimate of the number of larvae feeding on chipmunks in the signature matching analysis. Of the ticks that could be assigned to more than three species, 65% may have fed on chipmunks. These ticks were not assigned to any species, potentially causing the differences among the analyses. The estimates of the proportion of infected ticks that fed on mice and the two shrew species were very similar in the two analyses, supporting the accuracy of the estimates.
These analyses assume that host species act as different ecological niches for B. burgdorferi ss genotypes over time and space; a controversial assumption. Hanincova et al. (2006) recently suggested that every genomic lineage of B. burgdorferi ss is, in fact, a host generalist, as there is at least one example of each lineage in all of the vertebrate species investigated. The discrepancy between this report and that of Brisson & Dykhuizen (2004) is in the interpretation of the data, not in the data themselves. Both reports found that some B. burgdorferi ss lineages are transmitted to ticks at high frequencies; other lineages were also transmitted, but at very low frequencies (Brisson & Dykhuizen 2004; Hanincova et al. 2006). Brisson & Dykhuizen (2004) suggested that only the species that regularly transmit a genotype to feeding larvae were evolutionarily important for that genotype. The majority of the genotypes commonly found in the species examined in both studies were the same. The stability of transmission probabilities needs to be rigorously examined to generalize the findings presented here to other Lyme disease foci. Like the other species examined in these reports, the masked shrew transmits only a subset of genotypes to feeding larvae, supporting previous claims that each species represents a different pathogen niche (Brisson & Dykhuizen 2004). Additionally, the transmission probability distributions did not differ significantly among the individuals tested. However, these estimates came from only 66 infected ticks that were not evenly distributed (range=4–17) across six individuals. The transmission probability estimates from S. cinereus are not as certain as are those from other species where more individuals were assayed. The estimates of the number of larvae that feed on each species or the proportion of the infected ticks that fed on each species are not sensitive to errors in transmission probabilities measurements (table 3), instilling confidence in the estimates of Pj and reservoir potential.
Our studies at a representative site in the centre of the northeastern Lyme disease endemic zone indicate that shrews are important B. burgdorferi ss reservoirs, with the two species together feeding and infecting slightly more than half of the population of infected nymphs. A previous study reported that short-tailed shrews are rarely parasitized by larval ticks, suggesting that B. brevicauda and possibly other shrew species play only a small role in the Lyme disease system in the northeastern USA (Telford et al. 1990). However, Telford et al. (1990) estimated tick burdens by counting visible larvae (less than 0.5 mm) on the heads of field-caught animals. Our experience with hundreds of field-caught shrews is that larvae visible to the observer constitute a small fraction of the actual larval load. Counting fewer than five larvae on individual shrews that contributed more than 50 fed larvae over 72 h in the laboratory (LoGiudice et al. 2003; Brisson & Dykhuizen 2004) is typical at our sites (D. Brisson 2002–2006, unpublished data).
All other vertebrate species, including squirrels, deer, voles, raccoons, opossums and skunks, are poor reservoirs for B. burgdorferi ss (LoGiudice et al. 2003; Brisson & Dykhuizen 2004; Hanincova et al. 2006). Our estimates suggest that these species collectively feed half of the larval population, although the analyses employed in this study cannot quantify the importance of each species individually. These species represent ‘dilution hosts’, animals that decrease the prevalence of B. burgdorferi ss in ticks by feeding many but infecting few larval ticks (LoGiudice et al. 2003). Ground-foraging songbirds, however, may not be a dilution host species. LoGiudice et al. (2003) found LRC of a group of ground-dwelling songbirds, but other studies describe somewhat higher values for some species (Rand et al. 1998; Richter et al. 2000; Ginsberg et al. 2005). Birds are important reservoirs for species in the B. burgdorferi sensu lato complex in the European Lyme disease system and may play a similar role in the northeastern USA (Battaly & Fish 1993; Gern & Humair 2002). Future investigations should make every effort to assess the role of ground-foraging birds in the northeastern Lyme disease system.
A recent long-term study conducted at these same sites compared the importance of climate, deer, mice and chipmunks in determining abundance and B. burgdorferi-infection prevalence of blacklegged ticks (Ostfeld et al. 2006a). Statistical models that included prior abundance of mice and chipmunks had dramatically greater explanatory power than did models that included climatic variables or deer abundance. However, only 35–40% of inter-annual variation in abundance of nymphal ticks was explained by prior rodent abundance, and less than 5% of the variation in nymphal infection prevalence was explained by rodent abundance, suggesting that other factors are important. The Ostfeld et al. (2006a) study did not incorporate inter-annual variation in shrew abundance, largely because estimating shrew density from standard live-trapping protocols is extremely difficult due to low recapture rates. Rodent abundance at these sites is driven largely by variable acorn production (Ostfeld et al. 2006a), but the population dynamics of insectivorous shrews are probably independent of acorns and largely independent of the two rodent species. In herbaceous habitats of Illinois, B. brevicauda abundance fluctuates three- to fourfold among years and is correlated with neither syntopic rodents nor any other measured extrinsic factor (Getz et al. 2004). The magnitude of B. brevicauda population fluctuations was similar at a deciduous forest site and was similarly uncorrelated with extrinsic, abiotic variables (Lima et al. 2002). Neither the patterns of population dynamics nor the factors that might cause inter-annual fluctuations in masked shrews have been well characterized. We suggest that understanding the population dynamics of these two shrew species would contribute strongly to both prediction and mitigation of Lyme disease risk.
Short-tailed shrews often thrive in fragmented or otherwise human-disturbed landscapes (Pagels et al. 1994; Nagorsen 1996; Brack 2006). A dilution effect model including B. brevicauda as well as mice as omnipresent species might in some cases be a more realistic representation of nature than one that includes only mice (Ostfeld & Keesing 2000a,b; Schmidt & Ostfeld 2001; LoGiudice et al. 2003). Including shrews and mice as the primary reservoirs in dilution effect models increases the complexity of previous models but does not necessarily change the basic tenets. Heavily disturbed areas such as some suburban landscapes where only mice and shrews are present will have a lower nymphal infection prevalence than areas with only mice, as shrews infect a smaller proportion of larvae that feed upon them than do mice. However, regions with both taxa will probably have a greater abundance of nymphs, and of infected nymphs, due to greater blood meal and pathogen host densities. In diverse communities, the majority of larval blood meals occur on dilution hosts (figure 1, table 2) reducing the prevalence of B. burgdorferi ss below what is expected in regions with only shrews and mice, as predicted by the dilution effect (LoGiudice et al. 2003). Dilution hosts, seldom transmit B. burgdorferi ss to ticks, but are important for the maintenance of the tick vector of this pathogen, a fundamental part of the B. burgdorferi ss transmission cycle (Burgdorfer et al. 1982).
Like many other zoonotic diseases (Taylor et al. 2001; Reed et al. 2003; Herrera et al. 2004), Lyme disease risk is influenced by several important natural reservoirs and is not tied closely to the dynamics of any single reservoir species. Identification of the significant transmission routes of B. burgdorferi ss is essential for effective management of the Lyme disease epidemic. In most cases, Lyme disease control strategies using B. burgdorferi ss vaccines or acaricides delivered mainly or exclusively to white-footed mice have resulted only in modest reductions in the number or infection prevalence of ticks (Dolan et al. 2004; Tsao et al. 2004; Hornbostel et al. 2005; Ostfeld et al. 2006b). Our results suggest that only limited success will accompany control strategies aimed at hosts if shrews are not included as targets for mitigation. Our analyses predicts approximately a 25% reduction in the prevalence of B. burgdorferi ss in nymphs after vaccinating all mice in a natural host community, as approximately 25% of infected ticks acquired B. burgdorferi ss from a mouse (table 1). This figure is similar to those found in four field trials demonstrating an operative mouse vaccination programme (approx. 23.5%; Tsao et al. 2004), highlighting the benefits of ecological knowledge for public health. Management strategies that focus on the primary reservoir, while significantly reducing the nymphal infection prevalence in a statistical sense (Tsao et al. 2004), might fail to reduce the human disease risk in a practical sense. A mitigation strategy that targets mice, chipmunks and both shrew species would reduce the proportion of nymphs carrying B. burgdorferi ss by 90% (figure S3 in the electronic supplementary material).
Acknowledgments
We thank Kelly Oggenfuss for brilliant work at IES, Mark Omura for Sorex shrew identification and Kathleen LoGiudice for helpful suggestions. This work was supported by NIHPHS grant no. GM60731, NIH grant R01AI053109 and NSF grant DEB 0444585.
Supplementary Material
Supplementary material containing Sorex shrew capture and husbandry methods, inverse model output, and predictions of the outcome of interventions
References
- Anderson J.F. Mammalian and avian reservoirs for Borrelia burgdorferi. Ann. NY Acad. Sci. 1988;539:180–191. doi: 10.1111/j.1749-6632.1988.tb31852.x. doi:10.1111/j.1749-6632.1988.tb31852.x [DOI] [PubMed] [Google Scholar]
- Anderson J.M, Norris D.E. Genetic diversity of Borrelia burgdorferi sensu stricto in Peromyscus leucopus, the primary reservoir of Lyme disease in a region of endemicity in southern Maryland. Appl. Environ. Microbiol. 2006;72:5331–5341. doi: 10.1128/AEM.00014-06. doi:10.1128/AEM.00014-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnason A.N, Schwarz C.J. Using POPAN-5 to analyse banding data. Bird Study. 1999;46:157–168. [Google Scholar]
- Battaly G.R, Fish D. Relative importance of bird species as hosts for immature Ixodes dammini (Acari: Ixodidae) in a suburban residential landscape of southern New York State. J. Med. Entomol. 1993;30:740–747. doi: 10.1093/jmedent/30.4.740. [DOI] [PubMed] [Google Scholar]
- Brack V., Jr Short-tailed Shrews (Blarina brevicauda) exhibit unusual behavior in an urban environment. Urban Hab. 2006;4:127–132. [Google Scholar]
- Brisson D, Dykhuizen D.E. ospC diversity in Borrelia burgdorferi: different hosts are different niches. Genetics. 2004;168:713–722. doi: 10.1534/genetics.104.028738. doi:10.1534/genetics.104.028738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brisson D, Dykhuizen D.E. A modest model explains the distribution and abundance of Borrelia burgdorferi strains. Am. J. Trop. Med. Hyg. 2006;74:615–622. [PMC free article] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour A.G, Hayes S.F, Benach J.L, Grunwaldt E, Davis J.P. Lyme disease—a tick-borne spirochetosis. Science. 1982;216:1317–1319. doi: 10.1126/science.7043737. doi:10.1126/science.7043737 [DOI] [PubMed] [Google Scholar]
- CDC. Lyme disease—United States, 2001–2002. Morb. Mortal. Wkly Rep. 2004;53:365–369. [PubMed] [Google Scholar]
- Deblinger R.D, Rimmer D.W. Efficacy of a permethrin-based acaricide to reduce the abundance of Ixodes dammini (Acari: Ixodidae) J. Med. Entomol. 1991;28:708–711. doi: 10.1093/jmedent/28.5.708. [DOI] [PubMed] [Google Scholar]
- Derdakova M, Dudioak V, Brei B, Brownstein J.S, Schwartz I, Fish D. Interaction and transmission of two Borrelia burgdorferi sensu stricto strains in a tick-rodent maintenance system. Appl. Environ. Microbiol. 2004;70:6783–6788. doi: 10.1128/AEM.70.11.6783-6788.2004. doi:10.1128/AEM.70.11.6783-6788.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan M.C, Maupin G.O, Schneider B.S, Denatale C, Hamon N, Cole C, Zeidner N.S, Stafford K.C. Control of immature Ixodes scapularis (Acari: Ixodidae) on rodent reservoirs of Borrelia burgdorferi in a residential community of southeastern Connecticut. J. Med. Entomol. 2004;41:1043–1054. doi: 10.1603/0022-2585-41.6.1043. [DOI] [PubMed] [Google Scholar]
- Earnhart C.G, Buckles E.L, Dumler J.S, Marconi R.T. Demonstration of OspC type diversity in invasive human Lyme disease isolates and identification of previously uncharacterized epitopes that define the specificity of the OspC murine antibody response. Infect. Immun. 2005;73:7869–7877. doi: 10.1128/IAI.73.12.7869-7877.2005. doi:10.1128/IAI.73.12.7869-7877.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitch H, Sandidge L. Ecology of the opossum on a natural area in northeastern Kansas. Univ. Kansas Publ. Musuem Nat. Hist. 1953;7:305–338. [Google Scholar]
- Gern L, Humair P.-F. CABI Publishing; New York, NY: 2002. Ecology of Borrelia burgdorferi sensu lato in Europe. Lyme borreliosis: biology, epidemiology, and control. [Google Scholar]
- Getz L.L. A 14-year study of Blarina brevicauda populations in east central Illinois. J. Mammal. 1989;70:58–66. doi:10.2307/1381669 [Google Scholar]
- Getz L, Hofmann J, McGuire B, Oli M. Population dynamics of the northern short-tailed shrew, Blarina brevicauda: insights from a 25-year study. Can. J. Zool. 2004;82:1679–1686. doi:10.1139/z04-166 [Google Scholar]
- Ginsberg H.S, Buckley P.A, Balmforth M.G, Zhioua E, Mitra S, Buckley F.G. Reservoir competence of native north American birds for the Lyme disease spirochete, Borrelia burgdorferi. J. Med. Entomol. 2005;42:445–449. doi: 10.1603/0022-2585(2005)042[0445:RCONNA]2.0.CO;2. doi:10.1603/0022-2585(2005)042[0445:RCONNA]2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hanincova K, Kurtenbach K, Diuk-Wasser M, Brei B, Fish D. Epidemic spread of Lyme borreliosis, northeastern United States. Emerg. Infect. Dis. 2006;12:604–611. doi: 10.3201/eid1204.051016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera H.M, Davila A.M, Norek A, Abreu U.G, Souza S.S, D'Andrea P.S, Jansen A.M. Enzootiology of Trypanosoma evansi in Pantanal, Brazil. Vet. Parasitol. 2004;125:263–275. doi: 10.1016/j.vetpar.2004.07.013. doi:10.1016/j.vetpar.2004.07.013 [DOI] [PubMed] [Google Scholar]
- Hoffmann C.O, Gottschang J.L. Numbers, distribution, and movements of a raccoon population in a suburban residential community. J. Mammal. 1977;58:623–636. doi:10.2307/1380010 [Google Scholar]
- Holmes A, Sanderson G. Populations and movements of opossums in east-central Illinois. J. Wildl. Manage. 1965;29:287–295. doi:10.2307/3798433 [Google Scholar]
- Hornbostel V.L, Ostfeld R.S, Benjamin M.A. Effectiveness of Metarhizium anisopliae (Deuteromycetes) against Ixodes scapularis (Acari: Ixodidae) engorging on Peromyscus leueopus. J. Vector Ecol. 2005;30:91–101. [PubMed] [Google Scholar]
- Johns R, Ohnishi J, Broadwater A, Sonenshine D.E, De Silva A.M, Hynes W.L. Contrasts in tick innate immune responses to Borrelia burgdorferi challenge: immunotolerance in Ixodes scapularis versus immunocompetence in Dermacentor variabilis (Acari: Ixodidae) J. Med. Entomol. 2001;38:99–107. doi: 10.1603/0022-2585-38.1.99. [DOI] [PubMed] [Google Scholar]
- Lima M, Merritt J, Bozinovic F. Numerical fluctuations in the northern short-tailed shrew: evidence of non-linear feedback signatures on population dynamics and demography. J. Anim. Ecol. 2002;71:159–172. doi:10.1046/j.1365-2656.2002.00597.x [Google Scholar]
- LoGiudice K, Ostfeld R.S, Schmidt K.A, Keesing F. The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proc. Natl Acad. Sci. USA. 2003;100:567–571. doi: 10.1073/pnas.0233733100. doi:10.1073/pnas.0233733100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C.C. Mortality of unfed nymphal Ixodes dammini (Acari: Ixodidae) in field exclosures. Environ. Entomol. 1993;22:82–87. [Google Scholar]
- Magnarelli L.A, Anderson J.F, Fish D. Transovarial transmission of Borrelia burgdorferi in Ixodes dammini (Acari:Ixodidae) J. Infect. Dis. 1987;156:234–236. doi: 10.1093/infdis/156.1.234. [DOI] [PubMed] [Google Scholar]
- Markowski D, Ginsberg H.S, Hyland K.E, Hu R.J. Reservoir competence of the meadow vole (Rodentia: Cricetidae) for the Lyme disease spirochete Borrelia burgdorferi. J. Med. Entomol. 1998;35:804–808. doi: 10.1093/jmedent/35.5.804. [DOI] [PubMed] [Google Scholar]
- Mather T.N, Mather M.E. Intrinsic competence of three ixodid ticks (Acari) as vectors of the Lyme disease spirochete. J. Med. Entomol. 1990;27:646–650. doi: 10.1093/jmedent/27.4.646. [DOI] [PubMed] [Google Scholar]
- Mather T.N, Wilson M.L, Moore S.I, Ribeiro J.M, Spielman A. Comparing the relative potential of rodents as reservoirs of the Lyme disease spirochete (Borrelia burgdorferi) Am. J. Epidemiol. 1989;130:143–150. doi: 10.1093/oxfordjournals.aje.a115306. [DOI] [PubMed] [Google Scholar]
- Merritt J. University of Pittsburg Press; Pittsburg, PA: 1987. Guide to mammals of Pennsylvania. [Google Scholar]
- Montgomery S, Whelan J, Mosby H. Bioenergetics of a woodlot gray squirrel population. J. Wildl. Manage. 1975;39:709–717. doi:10.2307/3800232 [Google Scholar]
- Mosby H.S. The influence of hunting on the population dynamics of a woodlot gray squirrel population. J. Wildl. Manage. 1969;33:59–73. doi:10.2307/3799650 [Google Scholar]
- Nagorsen D. UBC Press; Vancouver, BC: 1996. Opossums, shrews and moles of British Columbia: Royal British Columbia Museum handbook. [Google Scholar]
- Ostfeld R, Keesing F. The function of biodiversity in the ecology of vector-borne zoonotic diseases. Can. J. Zool. Rev. Can. De Zool. 2000a;78:2061–2078. doi:10.1139/cjz-78-12-2061 [Google Scholar]
- Ostfeld R.S, Keesing F. Biodiversity and disease risk: the case of Lyme disease. Conserv. Biol. 2000b;14:722–728. doi:10.1046/j.1523-1739.2000.99014.x [Google Scholar]
- Ostfeld R.S, Schauber E.M, Canham C.D, Keesing K, Jones C.G, Wolff J.O. Effects of acorn production and mouse abundance on abundance and Borrelia burgdorferi infection prevalence of nymphal Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001;1:55–64. doi: 10.1089/153036601750137688. doi:10.1089/153036601750137688 [DOI] [PubMed] [Google Scholar]
- Ostfeld R.S, Canham C.D, Oggenfuss K, Winchcombe R.J, Keesing F. Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biol. 2006a;4:e145. doi: 10.1371/journal.pbio.0040145. doi:10.1371/journal.pbio.0040145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostfeld R.S, Price A, Hornbostel V.L, Benjamin M.A, Keesing F. Controlling ticks and tick-borne zoonoses with biological and chemical agents. Bioscience. 2006b;56:383–394. doi:10.1641/0006-3568(2006)056[0383:CTATZW]2.0.CO;2 [Google Scholar]
- Pagels J, Uthus K, Duval H. The masked shrew, Sorex cinereus, in a relictual habitat of the southern Applachian Mountains. In: Merritt J, Kirkland G, Rose R, editors. Advances in the biology of shrews. Carnegie Museum of Natural History Special Publication. vol. 18. Carnegie Museum of Natural History; Pittsburgh, PA: 1994. pp. 103–109. [Google Scholar]
- Porco T.C. A mathematical model of the ecology of Lyme disease. Math. Med. Biol. 1999;16:261–296. doi:10.1093/imammb/16.3.261 [PubMed] [Google Scholar]
- Qiu W.G, Dykhuizen D.E, Acosta M.S, Luft B.J. Geographic uniformity of the Lyme disease spirochete (Borrelia burgdorferi) and its shared history with tick vector (Ixodes scapularis) in the northeastern United States. Genetics. 2002;160:833–849. doi: 10.1093/genetics/160.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand P.W, Lacombe E.H, Smith R.P, Jr, Rich S.M, Kilpatrick C.W, Dragoni C.A, Caporale D. Competence of Peromyscus maniculatus (Rodentia: Cricetidae) as a reservoir host for Borrelia burgdorferi (Spirochaetares: Spirochaetaceae) in the wild. J. Med. Entomol. 1993;30:614–618. doi: 10.1093/jmedent/30.3.614. [DOI] [PubMed] [Google Scholar]
- Rand P.W, Lacombe E.H, Smith R.P, Jr, Ficker J. Participation of birds (Aves) in the emergence of Lyme disease in southern Maine. J. Med. Entomol. 1998;35:270–276. doi: 10.1093/jmedent/35.3.270. [DOI] [PubMed] [Google Scholar]
- Reed K.D, Meece J.K, Henkel J.S, Shukla S.K. Birds, migration and emerging zoonoses: West Nile Virus, Lyme disease, Influenza A and enteropathogens. Clin. Med. Res. 2003;1:5–12. doi: 10.3121/cmr.1.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richens V. Numbers and habitat affinities of small mammals in northwestern Maine. Can. Field Nat. 1974;88:191–196. [Google Scholar]
- Richter D, Spielman A, Komar N, Matuschka F.R. Competence of American robins as reservoir hosts for Lyme disease spirochetes. Emerg. Infect. Dis. 2000;6:133–138. doi: 10.3201/eid0602.000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley S, Hadidian J, Manski D. Population density, survival, and rabies in raccoons in an urban national park. Can. J. Zool. 1998;76:1153–1164. doi:10.1139/cjz-76-6-1153 [Google Scholar]
- Sanderson G. Estimating opossum populations by marking young. J. Wildl. Manage. 1961;25:20–27. doi:10.2307/3796986 [Google Scholar]
- Schmidt K.A, Ostfeld R.S. Biodiversity and the dilution effect in disease ecology. Ecology. 2001;82:609–619. [Google Scholar]
- Schwan T.G, Kime K.K, Schrumpf M.E, Coe J.E, Simpson W.J. Antibody response in white-footed mice (Peromyscus leucopus) experimentally infected with the Lyme disease spirochete (Borrelia burgdorferi) Infect. Immun. 1989;57:3445–3451. doi: 10.1128/iai.57.11.3445-3451.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokal R.R, Rohlf F.J. W.H. Freeman and Company; New York, NY: 1995. Biometry. [Google Scholar]
- Taylor L.H, Latham S.M, Woolhouse M.E. Risk factors for human disease emergence. Phil. Trans. R. Soc. B. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. doi:10.1098/rstb.2001.0888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telford S.R, III, Mather T.N, Adler G.H, Spielman A. Short-tailed shrews as reservoirs of the agents of Lyme disease and human babesiosis. J. Parasitol. 1990;76:681–683. [PubMed] [Google Scholar]
- Thompson D.C. Social system of grey squirrel. Behaviour. 1978;64:305–328. [Google Scholar]
- Tsao J.I, Wootton J.T, Bunikis J, Luna M.G, Fish D, Barbour A.G. An ecological approach to preventing human infection: vaccinating wild mouse reservoirs intervenes in the Lyme disease cycle. Proc. Natl Acad. Sci. USA. 2004;101:18 159–18 164. doi: 10.1073/pnas.0405763102. doi:10.1073/pnas.0405763102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade-Smith J, Verts B. Mephitis mephitis. Mammal. Species. 1982;173:1–7. doi:10.2307/3503883 [Google Scholar]
- Wang I.N, Dykhuizen D.E, Qiu W, Dunn J.J, Bosler E.M, Luft B.J. Genetic diversity of ospC in a local population of Borrelia burgdorferi sensu stricto. Genetics. 1999;151:15–30. doi: 10.1093/genetics/151.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker J, Hamilton W. Comstock Publishing Associates; Ithaca, NY: 1998. Mammals of the eastern United States. [Google Scholar]
- Wilson D.E, Reeder D.M. Smithsonian Institution Press; Washington, DC: 1993. Mammal species of the world. [Google Scholar]
- Wilson D, Ruff S. Smithsonian Institution Press; Washington, DC; London, UK: 1999. The smithsonian book of North American mammals. [Google Scholar]
- Woolhouse M.E.J, Gowtage-Sequeria S. Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material containing Sorex shrew capture and husbandry methods, inverse model output, and predictions of the outcome of interventions