Abstract
In 1868 Thomas Huxley first proposed that dinosaurs were the direct ancestors of birds and subsequent analyses have identified a suite of ‘avian’ characteristics in theropod dinosaurs. Ossified uncinate processes are found in most species of extant birds and also occur in extinct non-avian maniraptoran dinosaurs. Their presence in these dinosaurs represents another morphological character linking them to Aves, and further supports the presence of an avian-like air-sac respiratory system in theropod dinosaurs, prior to the evolution of flight. Here we report a phylogenetic analysis of the presence of uncinate processes in Aves and non-avian maniraptoran dinosaurs indicating that these were homologous structures. Furthermore, recent work on Canada geese has demonstrated that uncinate processes are integral to the mechanics of avian ventilation, facilitating both inspiration and expiration. In extant birds, uncinate processes function to increase the mechanical advantage for movements of the ribs and sternum during respiration. Our study presents a mechanism whereby uncinate processes, in conjunction with lateral and ventral movements of the sternum and gastral basket, affected avian-like breathing mechanics in extinct non-avian maniraptoran dinosaurs.
Keywords: Aves, theropod, uncinate processes, ventilation, gastralia
1. Introduction
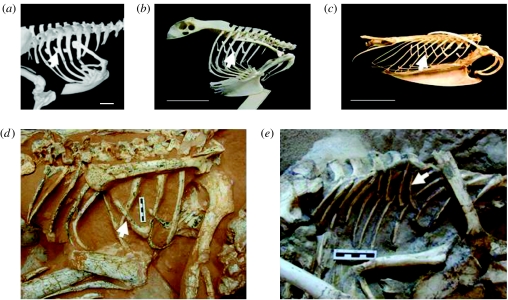
Uncinate processes extend from the caudal edge of the vertebral ribs. In modern birds (with the exception of the Sphenisciformes and Apteryx), the processes are ossified to the thoracic ribs and typically have a bell-shaped base and taper distally (figure 1a–c). Avian uncinate processes were originally interpreted as having a primary function in stiffening the rib cage and stabilizing the shoulder musculature (Bellairs & Jenkins 1960). However, recent work has demonstrated that uncinate processes are crucial ventilatory structures in birds, involved in both inspiration and expiration (Codd et al. 2005). Morphological adaptations including differences in wing shape, leg morphology and the rib cage are commonplace in birds with adaptations to different forms of locomotion. Interestingly, recent work has indicated that uncinate length also correlates with locomotor mode in birds: being short in walking (figure 1a); intermediate length in flying (figure 1b); and long in diving species (figure 1c; Tickle et al. 2007). The appendicocostal muscle attaches the uncinate process to the following rib and is active during inspiration in the giant Canada goose (Codd et al. 2005). The uncinate processes also act as a strut for the insertion of the external oblique muscle, which pulls the sternum dorsally during expiration (Codd et al. 2005).
Figure 1.
Uncinate processes (arrows) of (a) a running bird, the Cassowary (Casuaris casuaris), (b) a flying bird, the Eagle Owl (Bubo bubo), (c) a diving bird, the Razorbill (Alca torda); canonical variate analyses indicates that the uncinate processes are shorter in running, long in diving and intermediate in all other birds (Tickle et al. 2007), (d) AMNH 6517, (Oviraptor philoceratops), (e) MPD 100/25 (Velociraptor mongoliensis). Anterior is to the right in all figures. Scale bars, 5 cm. Photographs taken by J.R.C.
Recently, we have developed a geometric model to relate uncinate function to morphological differences associated with locomotor specializations in birds. Our geometric model indicates that uncinate processes act as levers which improve the mechanical advantage for the forward rotation of the dorsal ribs and therefore lowering of the sternum during respiration in birds (Tickle et al. 2007; figures 2–4). Given their lever arm action and site for muscle attachment, the length of the processes is functionally important as it will alter the mechanical advantage and area available for muscle attachment.
Figure 2.
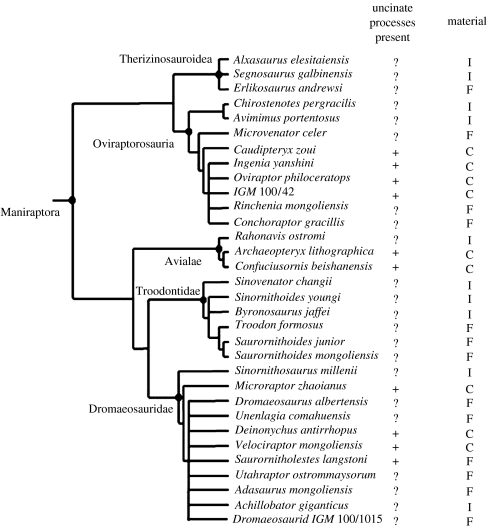
Summary cladogram of maniraptoran dinosaurs showing the occurrence of ossified uncinate processes. Uncinate processes: plus, ossified uncinate processes present; minus, ossified uncinate processes absent; question mark, presence of ossified uncinates unknown. Material: C, complete postcranial skeleton; I, incomplete postcranial skeleton; F, fragmentary postcranial skeleton. (Adapted from Clark et al. (2002).)
Figure 3.
Uncinate processes (arrows) of IVPP V13352 (Microraptor gui). Anterior is to the left. Scale bar, 13 cm. (Adapted with permission from Xu et al. (2003).)
Figure 4.
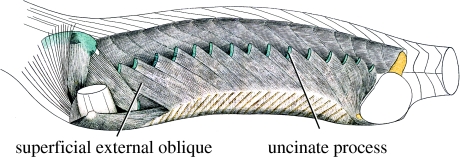
Hypaxial musculature of tuatara (Sphenodon punctatus) demonstrating insertion of external oblique muscle from the gastralia to the uncinate processes. Anterior is to the left. (Adapted with permission from Maurer (1896).)
The aim of this paper is to review the occurrence and morphology of uncinate processes in non-avian maniraptoran dinosaurs. In particular, we have examined the phylogenetic distribution of these processes to determine whether these are homologous structures. The wider distribution of uncinate processes and analogous structures will also be determined. Furthermore, the occurrence of these processes, in conjunction with movements of the sternum and a kinetic gastral basket, has reconstructed avian-like breathing mechanics in extinct non-avian maniraptoran dinosaurs.
2. Uncinate processes in non-avian maniraptoran dinosaurs
Recent discoveries have improved our understanding of the phylogenetic relationships of theropods and indicate that origin of Aves (Archaeopteryx to Neornithes: Padian 1998) is embedded within maniraptoran theropod dinosaurs (Clark et al. 2002; figure 2). Within non-avian maniraptoran dinosaurs, ossified uncinate processes are found in oviraptorids and many dromaeosaurs.
Oviraptor philoceratops (AMNH FR 6517) has four ossified uncinate processes present on the first four thoracic ribs (Clark et al. 1999; figure 1d). The processes extend from the caudal edge at the midpoint of the thoracic ribs and overlap the preceding ribs. The uncinate processes are flat, broad and bell shaped at the base, then taper distally and have a characteristic ‘L-shaped’ morphology, most closely resembling that of flying avian species (figure 1b). The processes on the first and fourth ribs are reduced in length and thickness. However, the uncinate processes on the second and third ribs exhibit both uniform geometry and morphology. The processes are not ossified to the ribs.
Five long uncinate processes are present on the left hand side of Velociraptor mongoliensis (MPD 100/25; Kielan-Jaworowska & Barsbold 1972; Norell & Makovicky 1999, 2004), extending from the caudal edge at the midpoint of the third to the seventh thoracic ribs to the edge of the second following rib (figure 1e). As described for Oviraptor, the processes are not ossified to the rib (figure 1e). In this taxon, the uncinate processes most closely resemble those of the diving avian species (figure 1c). The processes are flattened and uniform in morphology; they are thin and taper towards the tip. The uncinate process on the seventh rib is reduced in size and length and has been displaced from the thoracic rib. Rib scars indicate that the uncinate processes on the first and second thoracic ribs are missing.
The description of Deinonychus antirrhopus (Ostrom 1969) made no mention of uncinate processes; however, ‘probable ventral ribs’ were figured (see Ostrom 1969, fig. 52). A review of Deinonychus (YPM5246, YPM5247 and YPM5250) material has identified these ‘probable’ elements as uncinate processes (P. L. Manning 2006, personal observation). The ‘articular scars’ on Deinonychus ribs (Ostrom 1969) were almost certainly cartilaginous attachment sites between the thoracic rib and proximally expanded articular surface of each uncinate process. Deinonychus uncinates are typically bell shaped proximally, taper distally and were not ossified to the ribs. The uncinates may have been attached via cartilage as is suggested by corresponding roughened areas. The length of the uncinates indicates that they would have caudal–dorsally spanned several (2–4) ribs. It follows that gastral elements described in other non-avian maniraptoran theropods may also represent misidentified, unfused uncinate processes.
The smallest known non-avian theropods, Microraptor zhaoianus (V12330; Xu et al. 2000; Hwang et al. 2002) and Microraptor gui (IVPP V13352; Xu et al. 2003), are often well preserved in life positions and have uncinate processes (figure 3). The uncinate processes of Microraptor expand from the caudal edge of the ribs to a bell-shaped articulation with a distal tapering shaft and are homologous to, but much smaller than, those identified in this study for Deinonychus. Each process spans three ribs at an angle of 55° and was not fused to the ribs.
The occurrence of uncinate processes is patchy among the non-avian maniraptoran dinosaurs (figure 2). In the species so far described (Velociraptor, Microraptor, Deinonychus, Khaan and Citipati), the morphology of the uncinate processes indicates that they were attached via cartilage as shown by characteristic rough regions on both the ribs and the posterior articular surface of the processes. Cartilage does not readily fossilize as it has a lower structural density than bone (Lyman 1994) meaning the processes may have become detached and lost during disarticulation, weathering or scavenging of the skeleton. Given the diminutive size of these bones, it is also possible that they have been routinely overlooked during collection or simply not recognized as uncinate processes (e.g. Ostrom 1969). The cartilaginous attachment of the process to rib would also make disarticulation and mixing with gastralia elements potentially problematic for identification. Uncinate processes have also been described in some basal avian species, Confuciusornis (Zhonghe & Lianhai 2002) and may also be present in Archaeopteryx (Mayr et al. 2005; see figure 2b; P. L. Manning 2007, personal observation). Using parsimony, the presence of uncinates in early avian species, their presence in several non-avian maniraptoran groups and their phylogenetic distribution indicate that they are homologous structures.
3. Wider distribution of uncinate processes and analogous structures
Cartilaginous or ossified uncinate processes are reported in crocodiles (Hofstetter & Gasc 1969) and the tuatara (Romer 1956); however, their possible role as ventilatory structures has yet to be determined. Well-developed gastralia are also present in both crocodilians and tuatara and have been suggested to play some role during ventilation (Lambe 1917; Perry 1983; Carrier & Farmer 2000; Claessens 2004). Indeed, in tuatara (Sphenodon punctatus), the external oblique muscle originates at the margin of the gastral basket and inserts onto the base of the uncinate processes similar to that described in Aves (Maurer 1896; figure 4).
Rib flanges which are occasionally referred to as uncinate processes are also well described in early tetrapods (Clack 2002). For example, Eryops and Dissorophus possessed small triangular costal expansions on the thoracic ribs (Gregory 1951; Clack 2002). The general morphology of these structures and their location on the ribs, which get progressively more ventral as you move rostrally, are quite different from uncinate processes found in Aves and the non-avian Maniraptora. The ventilatory mechanics of early tetrapods are unclear; however, air gulping, buccal pumping and an early version of rib movement have all been suggested. Costal breathing is thought to have originated in early amniotes (Clack 2002; Perry & Sander 2004). The expanded ribs of early tetrapods may have been associated with the development of the transverse abdominal muscle which assists exhalation during aspiration breathing in tetrapods (Brainerd et al. 1993) or alternatively these processes are thought to function in the stiffening of the body during axial locomotion rather than serving any putative respiratory function (Clack 2002; J. Gauthier 2007, personal communication).
Some ornithischian dinosaurs (e.g. Thescelosaurus) possessed deep, plate-like intercostal structures attaching successive thoracic ribs (Fisher et al. 2000; see figure 1). Uncinates are morphologically distinct structures either fused to the thoracic ribs (Aves) or connected via cartilage (Non-Avian Maniraptora), while the multiple (often more than one per rib) structures observed in ornithischian dinosaurs consist of proximally and distally attached cartilaginous sheets connecting rib to rib. The structures observed in ornithischian dinosaurs are not likely to be homologous to avian uncinates. Furthermore, skeletal pneumatization is absent in ornithischian dinosaurs suggesting that they possessed a less ‘bird-like’ respiratory system than the theropods (Perry & Sander 2004). However, the cartilaginous plates of ornithischian dinosaurs might have functioned as passive elastic structures assisting expiration, with the recoil energy from inhalation reducing the volume of the lungs by pulling the rib cage back to a neutral position.
4. Reconstructing the breathing mechanics of non-avian maniraptoran dinosaurs
Indirect evidence must be used to reconstruct the mechanics of breathing in extinct dinosaurs as there are no fossilized soft tissues or lungs. Both crocodiles and birds have a greater diffusing capacity in more proximal than distal regions of their lungs. Therefore, dinosaurs, being phylogenetically bracketed by these two groups (Witmer 1995), have been suggested to also possess a highly heterogeneous lung structure (Perry & Sander 2004). Furthermore, the pattern of regional postcranial pneumatization in theropods is similar to modern birds and has been used as evidence of the presence of a cervical and abdominal air-sac system in non-avian theropods (Britt 1997; Burnham et al. 2000; Perry 2001; O'Connor & Claessens 2005). Taken together, these suggest that a flow through respiratory system is a general theropod characteristic (O'Connor & Claessens 2005). The implications of a putative air-sac system in theropods are that these were highly active animals.
The morphology of the uncinate processes in extant bipedal flightless birds is very different from that of extinct bipedal non-avian maniraptoran dinosaurs (figure 1). The uncinate processes in non-avian maniraptoran dinosaurs are not reduced as in running birds but are of intermediate to long lengths and resemble those of the flying or diving birds (figure 1). Running avian species lack the large breast musculature associated with flight, and therefore have no need for the lever-arm action provided by the uncinate processes to move a large muscle mass. The relatively long processes described for non-avian maniraptoran dinosaurs therefore suggest that an improved mechanical advantage, as demonstrated in extant avian species (Tickle et al. 2007), is functionally important. Birds ventilate their air sacs by moving the sternum in a dorsal–ventral plane to facilitate the bellows-like movement of air into and out of the air sacs. Non-avian maniraptoran dinosaurs lack a keeled sternum, but have well-developed gastralia in the ventral belly wall (Claessens 2004), and ossified sterna, attached to the distal ribs by large ossified sternal ribs. Gastralia have been postulated to play some role in respiration by preventing the inward movement of the belly wall (Perry 1983; Claessens 2004). However, more recently the gastral basket has been hypothesized to play a dynamic role in archosaur ventilation (Carrier & Farmer 2000).
The gastralia of non-avian maniraptoran theropods are highly derived, cross the midline and articulate with two gastralia from the opposite side of the body. This arrangement constitutes a lever arm which through association of hypaxial musculature could have narrowed or widened the cuirassal basket (Carrier & Farmer 2000). If the medial ends of the gastralia were drawn caudally by the ischiotruncus muscle, lateral and ventral movement of the belly wall would occur. The ischium functioned as the origin for the ischiotruncus muscle and the expanded foot of the pubis acted to direct the force of the muscle (Carrier & Farmer 2000). In modern birds, the external oblique muscle inserts onto the base of the uncinate processes from the sternum and the process acts as a strut to pull the sternal mass dorsally during expiration (Codd et al. 2005). A similar avian-like arrangement of the musculature in non-avian maniraptoran dinosaurs, whereby the external oblique pulled in a primarily dorsal direction, would have provided an attachment site for the insertion of an external oblique muscle originating at the edges of the gastral basket and sternum. This muscle, in conjunction with sternal movements and the narrowing and widening of the gastralia affected by the rectus and ischiotruncus, would have provided a mechanism for facilitating the bellows-like movement of air through a putative air-sac system.
Our study indicates that the presence of uncinate processes, coupled with specialized gastralia, sterna and pelvic girdles, provides a mechanism for facilitating avian-like breathing mechanics in non-avian maniraptoran dinosaurs.
Acknowledgments
We thank Rinchen Barsbold and Khishigjav Tsogtbaatar (Mongolian Academy of Science) for access and permission to study Velociraptor MPD 100/25. Carl Mehling (American Museum of Natural History) for facilitating access to Oviraptor specimens. Martin Sander, Bernhard Misof, Bill Sellers, Dave Carrier, Leon Claessens, Phil Currie and Dona Boggs for their useful discussions. We also thank Jacques Gauthier, Walter Joyce and Dan Brinkman for access to the Yale Peabody collections and helpful discussions. This work was funded by the Deutsche Forschungsgemeinschaft (DFG 721/1) and the University of Manchester.
References
- Bellairs A, Jenkins C.R. The skeleton of birds. In: Marshall A.J, editor. Biology and comparative physiology of birds. Academic Press; New York, NY: 1960. pp. 241–300. [Google Scholar]
- Burnham, D. A., Derstler, K. L., Currie, P. J., Bakker, R. T., Zhou, Z. & Ostrom, J. H. 2000 Remarkable new birdlike dinosaurs (Theropoda: Maniraptora) from the Upper Cretaceous of Montana University of Kansas Paleontological Contributions, no. 13, pp. 1–14. Lawrence, KS: The University of Kansas.
- Brainerd E.L, Ditelberg J.S, Bramble D.M. Lung ventilation in salamanders and the evolution of vertebrate air-breathing mechanisms. Biol. J. Linn. Soc. 1993;49:163–183. doi:10.1006/bijl.1993.1028 [Google Scholar]
- Britt B.B. Postcranial pneumaticity. In: Currie P.J, Padian K, editors. Encylopedia of dinosaurs. Academic Press; San Diego, CA: 1997. pp. 590–593. [Google Scholar]
- Carrier D.R, Farmer C.G. The integration of ventilation and locomotion in archosaurs. Am. Zool. 2000;40:87–100. doi:10.1668/0003-1569(2000)040[0087:TIOVAL]2.0.CO;2 [Google Scholar]
- Clack J.A. Indiana Press; Indianapolis, IN: 2002. Gaining ground: the origin and evolution of terapods. [Google Scholar]
- Claessens L.P.A.M. Dinosaur gastralia: origin, morphology and function. J. Vertebr. Paleontol. 2004;24:89–106. doi:10.1671/A1116-8 [Google Scholar]
- Clark J.M, Norell M.A, Chiappe L. An oviraptoid skeleton from the Late Cretaceous of Ukhaa Tolgod, Mongolia, preserved in an avian-like brooding position over an oviraptoid nest. Am. Mus. Novit. 1999;3265:1–36. [Google Scholar]
- Clark J.M, Norell M.A, Makovicky P.J. Cladistic approaches to the relationships of birds to other theropod dinosaurs. In: Chiappe L.M, Witmer L.M, editors. Mesozoic birds: above the heads of dinosaurs. University of California Press; London, UK: 2002. pp. 31–61. [Google Scholar]
- Codd J.R, Boggs D.F, Perry S.F, Carrier D.R. Activity of three muscles associated with the uncinate processes of the giant Canada goose (Branta canadensis maximus) J. Exp. Biol. 2005;208:849–857. doi: 10.1242/jeb.01489. doi:10.1242/jeb.01489 [DOI] [PubMed] [Google Scholar]
- Fisher P.E, Russell D.A, Stoskopf M.K, Barrick R.E, Hammer M, Kuzmitz A.A. Cardiovascular evidence for an intermediate or higher metabolic rate in an ornithischian dinosaur. Science. 2000;288:503–505. doi: 10.1126/science.288.5465.503. doi:10.1126/science.288.5465.503 [DOI] [PubMed] [Google Scholar]
- Gregory W.K. MacMillan Press; New York, NY: 1951. Evolution emerging. [Google Scholar]
- Hofstetter R, Gasc J.-P. Vertebrae and ribs of modern reptiles. In: Gans C, Bellairs A.d'A, Parsons T.S, editors. Biology of the reptilia. Morphology A. vol. 1. Academic Press; London, UK: 1969. pp. 201–310. [Google Scholar]
- Hwang S.H, Norell M.A, Qiang J, Keqin G. New specimens of Microraptor zhaoianus (Theropoda: Dromaeosauridae) from North-eastern China. Am. Mus. Novit. 2002;3381:1–44. doi:10.1206/0003-0082(2002)381<0001:NSOMZT>2.0.CO;2 [Google Scholar]
- Kielan-Jaworowska A, Barsbold R. Narrative of the Polish–Mongolian palaeontological expeditions 1967–1971. Paleontol. Polon. 1972;27:5–13. [Google Scholar]
- Lambe L.M. New genera and species from the Belly River Series (Mid-Cretaceous) Contrib. Can. Palaeontol. Geol. Surv. 1917;3:25–81. [Google Scholar]
- Lyman, R. L. 1994 Structure and quantification of vertebrate skeletons. In Vertebrate taphonomy, p. 79. Cambridge, UK: Cambridge University Press.
- Maurer, F. 1896 Die ventrale Rumpfmuskulatur einiger Reptilien. Festschrift zum 8. Geburtstage von Carl Gegenbaur Engelmann, Leipzig. 1–76.
- Mayr G, Pohl B, Peter D.S. A well preserved Archaeopteryx specimen with theropod features. Science. 2005;310:1483–1486. doi: 10.1126/science.1120331. doi:10.1126/science.1120331 [DOI] [PubMed] [Google Scholar]
- Norell M.A, Makovicky P.J. Important features of the dromeosaurid skeleton II: information from newly collected specimens of Velociraptor mongoliensis. Am. Mus. Novit. 1999;3282:1–45. [Google Scholar]
- Norell M.A, Makovicky P.J. Dromaeosauridae. In: Weishampel B, Dodson P, Osmolska H, editors. The Dinosauria. 2nd edn. University of California Press; Berkley: 2004. pp. 196–209. [Google Scholar]
- O'Connor P.M, Claessens L.P.A.M. Basic avian pulmonary design and flow-through ventilation in non-avian theropod dinosaurs. Nature. 2005;436:253–256. doi: 10.1038/nature03716. doi:10.1038/nature03716 [DOI] [PubMed] [Google Scholar]
- Ostrom J.H. Osteology of Deinonychus antirrhopus, an unusual theropod from the Lower Cretaceous of Montana. Peabody Nat. Hist. Bull. 1969;30:1–165. [Google Scholar]
- Padian K. When is a bird not a bird? Nature. 1998;393:729–730. doi:10.1038/31576 [Google Scholar]
- Perry, S. F. 1983 Reptilian lungs. In Functional anatomy and evolution Berlin, Germany: Springer. [PubMed]
- Perry S.F. Functional morphology of the reptilian and avian respiratory systems and its implications for theropod dinosaurs. In: Gauthier J, Gall L.F, editors. New perspectives on the origin and early evolution of birds. Peabody Museum of Natural History Yale University; New Haven, CT: 2001. pp. 429–441. [Google Scholar]
- Perry S.F, Sander P.M. Reconstructing the evolution of the respiratory apparatus in tetrapods. Resp. Physiol. Neurobiol. 2004;144:125–139. doi: 10.1016/j.resp.2004.06.018. doi:10.1016/j.resp.2004.06.018 [DOI] [PubMed] [Google Scholar]
- Romer, A. S. 1956 The axial skeleton. In Osteology of the reptiles Chicago, IL: University of Chicago Press.
- Tickle P.G, Ennos A.R, Lennox L.E, Perry S.F, Codd J.R. Functional significance of the uncinate processes in birds. J. Exp. Biol. 2007 doi: 10.1242/jeb.008953. doi:10.1242/jeb008953 [DOI] [PubMed] [Google Scholar]
- Witmer L.M. The extant phylogenetic bracket and the importance of reconstructing soft tissue in fossils. In: Thomason J.J, editor. Functional morphology in vertebrate palaeontology. Cambridge University Press; Cambridge, UK: 1995. pp. 19–33. [Google Scholar]
- Xu X, Zhou Z, Wang X. The smallest known non-avian theropod dinosaur. Nature. 2000;408:705–708. doi: 10.1038/35047056. doi:10.1038/35047056 [DOI] [PubMed] [Google Scholar]
- Xu X, Zhou Z, Wang X, Kuang X, Zhang F, Du X. Four-winged dinosaurs from China. Nature. 2003;421:335–340. doi: 10.1038/nature01342. doi:10.1038/nature01342 [DOI] [PubMed] [Google Scholar]
- Zhonghe Z, Lianhai H. The discovery and study of mesozoic birds in China. In: Chiappe L.M, Witmer L.M, editors. Mesozoic birds: above the heads of dinosaurs. University of California Press; Los Angeles, CA: 2002. pp. 171–173. [Google Scholar]