Abstract
It has been reported that wild capuchin monkeys exhibit several group-specific behavioural traditions. By contrast, experiments have found little evidence for the social learning assumed necessary to support such traditions. The present study used a diffusion chain paradigm to investigate whether a novel foraging task could be observationally learned by capuchins (Cebus apella) and then transmitted along a chain of individuals. We used a two-action paradigm to control for independent learning. Either of two methods (lift or slide) could be used to open the door of a foraging apparatus to retrieve food. Two chains were tested (N1=4; N2=5), each beginning with an experimenter-trained model who demonstrated to a partner its group-specific method for opening the foraging apparatus. After the demonstration, if the observer was able to open the apparatus 20 times by either method, then it became the demonstrator for a new subject, thus simulating the spread of a foraging tradition among ‘generations’ of group members. Each method was transmitted along these respective chains with high fidelity, echoing similar results presently available only for chimpanzees and children. These results provide the first clear evidence for faithful diffusion of alternative foraging methods in monkeys, consistent with claims for capuchin traditions in the wild.
Keywords: social learning, tradition, culture, primates, diffusion chains, Cebus apella
1. Introduction
In the last 20 years, there has been a major expansion in the study of social learning in animals, driven principally by the study of behavioural traditions in the wild, and experimental analyses of the learning process, undertaken mostly in the laboratory (Galef & Giraldeau 2001; Fragaszy & Perry 2003; Galef & Heyes 2004; Perry 2006). Social learning includes all those processes whereby individuals acquire new behaviour or information about their environment through observation or interaction with others, or the results of their actions. This may give rise to the group-level phenomenon of local traditions or cultures, in which case the social learning is often referred to as ‘cultural transmission’. Understanding such learning is important for evolutionary biology generally, because it provides an alternative transmission system to genetics that can powerfully shape behavioural evolution (Boyd & Richerson 1985; Whiten 2005). At more specific levels, studies of animal social learning and traditions have become influential in behavioural ecology (Danchin et al. 2004), ethology (Fragaszy & Perry 2003), anthropology (Perry 2006) and comparative psychology (Shettleworth 2001; Hurley & Chater 2005).
This body of work has provided increasing evidence for social learning and traditions among fishes, birds and mammals (Brown & Laland 2006; Learning & Behavior, whole issue 32 (1) 2004; Stanley et al. in press). However, the traditions described in most species studied tend to be limited to single behaviour patterns, such as pine-cone opening in black rats (Aisner & Terkel 1992; Terkel 1996). By contrast, in a small number of primate species that have been the subjects of long-term field study, multiple traditions have been described, which define relatively complex local ‘cultures’ that have been suggested to be somewhat more comparable to the multifarious nature of human culture (chimpanzees: Goodall 1973, Nishida et al. 1983, Whiten et al. 1999; orangutans: van Schaik et al. 2003; Japanese macaques: Leca et al. 2007; see Whiten & van Schaik (2007) for a review). Recent studies of capuchin monkeys (Cebus spp.) have provided the richest of such information for any monkey, extending to several forms of social conventions including finger-sniffing and dyadic games, and locally varying types of foraging behaviour that include nut-cracking and fruit-processing (Ottoni & Mannu 2001; Panger et al. 2002; Perry et al. 2003).
These reports rely on circumstantial evidence that genetic and environmental influences are unlikely to be responsible for the appearance of the group-specific behaviours described. However, the weakness of the field studies is that direct evidence implicating social learning, of the kind provided unambiguously through experimental manipulation of opportunities for social versus non-social learning, remains unavailable (Fragaszy 2003; Galef 2003; Laland & Hoppitt 2003). Accordingly, researchers have turned to laboratory experiments to complete studies of social learning that complement the field research.
The majority of such experiments with capuchin monkeys have converged on a conclusion that appears at odds with that drawn from the field studies: that capuchin monkeys are not imitators and that the limited transmission of information-recorded results from simpler social learning mechanisms such as social facilitation or localized stimulus enhancement, in which attention is merely drawn to relevant stimuli (Visalberghi & Fragaszy 1990, 2002). The authors of these studies have interpreted them as supporting the conclusion that monkeys do not imitate or learn from one another; rather, they simply learn with each other (Fragaszy & Visalberghi 2001; Bonnie & de Waal 2007), the presence of a conspecific merely facilitating an individual's ability to learn independently. The results of numerous experimental studies (Fragaszy & Visalberghi (2001, 2004) review over 30 studies) therefore appear in conflict with the inference of field researchers that group-specific behaviours are culturally transmitted in capuchin monkeys, because processes as simple as stimulus enhancement would be insufficient to generate the behavioural variants documented in wild capuchins, which concern particular foraging and social behaviours rather than preferences for objects or locations.
These social learning experiments, however, have been based on dyadic tests in which a single observer watches a single, trained model (Adams-Curtis & Fragaszy 1995; Fredman & Whiten in press; Coussi-Korbel & Fragaszy 1995; and see reviews by Visalberghi & Fragaszy (1990, 2002)). This is a limited paradigm for the study of culture, which requires the spread of novel forms of behaviour through a group. Our study therefore aimed to bridge the gap between the dyadic experimental studies of social learning and the population-level cultural phenomena inferred in the wild, by investigating whether brown capuchin monkeys (Cebus apella) are capable of transmitting a novel foraging task along a chain of individuals. Moreover, we applied a two-action paradigm (Dawson & Foss 1965; Galef et al. 1986), which controls for individual learning by having each of two alternative foraging methods performed by an initial model in front of a naive subject. The particular two-action design of this study also controls for localized stimulus enhancement by having both of the alternative, modelled foraging methods focused on the same locus of the task (the handle of a door, which can either be lifted or slid open to retrieve food). This paradigm was further strengthened by testing three groups of individuals: one group for each method and a third control group not exposed to a demonstrator of either method.
To address the fidelity of information transfer and the ability of a group to maintain an experimentally introduced foraging behaviour beyond the original model, we employed a diffusion chain paradigm. The diffusion chain paradigm, like the game ‘telephone’, involves information being transferred from one individual to the next. Although at each step in the experiment, we are again testing only a dyad, in this diffusion paradigm there is a realistic possibility for the information to be corrupted, if it is not copied exactly. If the latter occurs, the original behaviour will not spread to become a tradition. Thus, the diffusion chain simulates one ‘thread’ through a series of potential cultural transmission events.
The diffusion chain paradigm was first used with humans (Bartlett 1932) and has more recently been employed in a still-small set of studies to test the transmission of foraging, food preferences and predator avoidance in fishes, birds and rats (Curio et al. 1978; Laland & Plotkin 1990, 1992; Laland & Williams 1998). Recently, the three-group, two-action paradigm used in the current study demonstrated high fidelity transmission of alternative foraging methods along diffusion chains involving up to six steps in chimpanzees (Pan troglodytes), as well as in human children (Horner et al. 2006). It should be noted that other diffusion paradigms exist, such as ‘open diffusion’ in which a model is introduced into a whole group (Kendal et al. 2005; Whiten et al. 2007). The merit of the ‘chain’ paradigm is that it allows the course of the transmission to be known and ‘cultural generations’ showing faithful replication to be accurately counted.
Given the apparent lack of imitation in monkeys, it remains unknown whether such transmission chains would be sustained in the capuchins we studied. In the light of the experimental studies summarized above, one might instead expect corruption to occur early, since capuchins may not copy the behavioural variants seeded in their chain. The field research, however, would suggest that transmission will be sustained. By employing the diffusion chain paradigm in conjunction with a two-action social learning task, it should be possible to gain further insight into the transmission processes that support group-specific cultural variation in capuchins.
2. Material and methods
(a) Subjects
Subjects were 4 male and 10 female brown capuchin monkeys ranging in age from 3 to 30 years (median age, 5.5 years; mean age, 9). They lived in a group of 20 individuals (6 males, 11 females and 3 infants) ranging in age from two months to an estimated 35 years, housed at the Centre de Primatologie of Université Louis Pasteur in France.
Monkeys were housed in an enclosure consisting of two indoor areas measuring 33 m2 in total and three interconnected outdoor areas measuring 45 m2 in total. The outdoor enclosures were connected by 1 m long tunnels that could be closed off using sliding doors. All tests were conducted in the first outdoor enclosure area where both subjects could move freely. A visual barrier was placed so as to prevent future test subjects from observing the test condition from the second enclosure. Each test pair was separated from their group for testing, but for not more than 30 min. They had ad libitum access to monkey chow and water and were never food deprived.
Subject pairs were selected based on observations made by the first author, focusing on social tolerance during grooming bouts and food interest interactions in pairs. The demonstrator for each test was slightly higher ranking than the observer monkey. This was done so that the model would be able to manipulate the device without being displaced by the observer. The rank difference, however, was small enough that the observer was tolerated by the model. Prior to the first test session, all pairs were given a ‘compatibility check’, to see whether they both could be presented with food without conflict or displacement. This was deemed important since observer subjects had the opportunity to move about the 15 m2 enclosure and avoid the model, if there was conflict.
(b) Materials
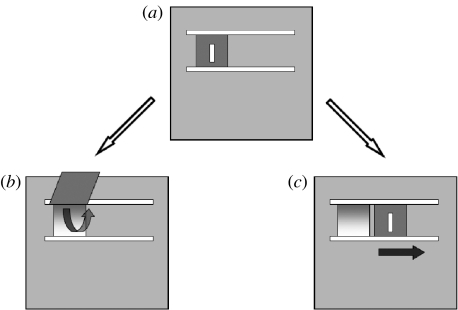
An ‘artificial fruit’ was constructed from Lexan and measured 28×28×28 cm. This was modelled on the device used by Horner et al. (2006), nicknamed the ‘Doorian fruit’ (henceforth ‘the Doorian’) scaled down appropriately for capuchin monkeys. The back of the Doorian was open to allow the experimenter to insert food items. Pieces of cereal were used as the food rewards. The door could be opened by either of two actions: (i) lifting or (ii) sliding (figure 1a–c). This two-action task controlled for stimulus and local enhancement because either method was possible at the same location.
Figure 1.
(a–c) Opening the ‘Doorian fruit’. (a) The front panel of the Doorian fruit apparatus was presented to subjects with the door in the closed, resting state. In the resting state, subjects could manipulate the door handle to open the apparatus by either (b) lifting or (c) sliding the door.
Our Doorian differed from that used by Horner et al. (2006) in three small but probably important ways for our subjects. First, the Doorian was elevated to monkey's shoulder level, allowing the capuchins to explore and manipulate the apparatus with both hands more naturally while in the seated position (the chimpanzee version was lower). Second, unlike the ape version, the slide method had no spring mechanism to return the door to the closed position, so preventing the monkeys from trapping their smaller fingers in the door. The Doorian had an opening in the back, which allowed the experimenter, sitting behind it, to reset the door to the closed position and to bait the device with food rewards. Third, a protruding door handle was added so that enough surface area existed for the monkeys to use their entire hand and wrist to open the door, since they appeared less capable of the grips employed by children and chimpanzees. In these ways, we contrived to make the task suitable for the known manipulative competencies of our subjects.
(c) Procedure
(i) Controls
To discover whether both methods were similarly difficult, four subjects were presented with the Doorian without any prior training or demonstrations. These individuals were given 15 min to manipulate the Doorian in order to extract food rewards. If the subject was successful using either method, the box was re-baited for 20 trials. A trial terminated with food retrieval from the box using either method, or if the monkey was unsuccessful, the control test ended after 15 min.
(ii) Model training
Each of two models was given three training sessions spread over 3 separate days, with each session consisting of 20, 30 and 40 trials, respectively. The range of 20–40 trials was employed in order to assess when a demonstrator became satiated. This occurred between 20 and 30 trials, with longer delays occurring between food retrieval attempts for trials 30–40. Because models and observers could not easily be separated after observations, satiating the demonstrator gave the observer the opportunity to go on to manipulate the Doorian. During the first training session, both the models were shown their respective method by having the experimenter open the box twice. Both the models were able to open the Doorian using the trained technique during the first training session after only two demonstrations.
The two models were selected based on rank. High-ranking models are most likely to be able to perform the task repeatedly without being displaced. Unfortunately, one of the models, the group's beta male, did not behave the same way when paired with some individuals as had been expected from the group context. After his training, he was presented with a ‘compatibility test’ to see whether he would allow a partner to take food from the experimenter in his presence, and he behaved antagonistically towards the partner. Therefore, this originally intended slide model was replaced by the alpha female, who performed the slide method during her control test. She performed 80% slide (i.e. 4 lifts/16 slides) during her control test, but then later performed 100% slide once exposed to training sessions. After three sessions of 20, 30 and 40 trials, she was considered a proficient model. Because there were a limited number of monkeys available for this study, there was one subject less in the slide group than in the lift (i.e. one trained model and four slide observers; one trained model and five lift observers).
(iii) Demonstration sessions and observer tests
Prior to testing, all potential test pairs were given a compatibility check during which food was presented to the pair in the test area. The experimenter showed two hands holding food rewards and then presented this food to both monkeys with hands apart. If the dominant allowed the subordinate to take food without aggression or major displacement, the pair was considered compatible for testing.
A ‘test’ consisted of two phases. In the first phase, a subject was given the opportunity to watch a demonstrator monkey open the Doorian and collect food for a minimum of 20 trials and a maximum of 40 trials. Subjects were considered ‘watching’ when facing the apparatus within arms reach of the demonstrator. A minimum of 20 trials was set so that the subjects had multiple opportunities to watch in close proximity to the demonstrator (figure 2). A maximum of 40 trials was set since subjects became satiated, variably, at some point between 20 and 40 trials. Once satiated, the model stopped monopolizing the Doorian, leaving the device available for manipulation by the observer monkey. In the second phase, the subject was allowed to manipulate the Doorian to search for food. If the observer was able to open the door by using either method and retrieve the food reward, the apparatus was re-baited for a total of 20 trials. Each observer who was able to open the Doorian became the demonstrator for the next test subject in the chain, whichever method they employed.
Figure 2.
Testing environment. Subjects accessed the Doorian through the mesh of their enclosure. Demonstrators either lifted or slid open the door to retrieve a cereal reward that was located on a tray behind the door. Observers watched in close proximity of the demonstrating monkey.
(d) Data collection and analysis
All tests were recorded with a Canon mini-DV video camera. The researcher also dictated the method used and whether the demonstration was watched by the observer, in case it was not clearly visible on film.
The number of lift and slide actions was recorded. The number of food-retrieval demonstrations observed by the subject, regardless of which action was performed, was recorded to assess the per cent of all demonstrations observed. Owing to the design of the task, coding of lift versus slide was unambiguous.
Owing to the small sample sizes, non-parametric statistics were used to compare the three groups on these measures.
3. Results
(a) Controls
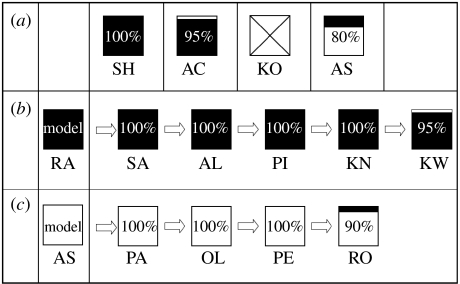
The control tests demonstrated that either method was possible for at least some capuchins to discover. Of the four controls, two performed the lift method with 100 and 95% success, respectively, and one performed 80% slide during their respective 20 trials. A fourth subject manipulated various places including the door handle on the Doorian, but was unable to open the door and did not extract food rewards (figure 3a).
Figure 3.
(a–c) Diffusion chain results. Each box represents one subject's performance (black area, per cent lift actions; white area, per cent slide actions; crossed area, no action) for (a) control, (b) lift and (c) slide groups. Codes under each box identify the subject. Arrows indicate the progression in the diffusion chain; each subject followed by an arrow was the model for the next subject in the chain.
(b) Observers
Subjects were all given between 20 and 40 demonstrations depending on how quickly the respective model became satiated. Subjects observed between 38 and 100% demonstrations performed by the model, with all subjects witnessing at least 10 trials (table 1). Subjects were considered ‘observing’ when facing the apparatus within arms reach of the demonstrator. All but two observers watched 80% or more of the demonstrations. The subject who watched 49% (KN) was initially pushed away by the model (PI) but quickly tolerated after the first seven trials. The second subject who watched 38% (KW) was tolerated by the model, but preferred to forage in the gravel of the test area for part of the demonstration phase.
Table 1.
The per cent of model demonstrations observed by the test subjects are presented by group in the order in which subjects appeared within their respective chain.
| lift group | slide group | ||
|---|---|---|---|
| subject | per cent trials observed (%) | subject | per cent trials observed (%) |
| Samir (SA) | 90 | Paola (PA) | 95 |
| Alila (AL) | 80 | Olive (OL) | 85 |
| Pistou (PI) | 100 | Petula (PE) | 100 |
| Kinika (KN) | 49 | Rosy (RO) | 100 |
| Kiwi (KW) | 38 | ||
The number of food retrievals using either lift or slide was tallied for each of the 20 trials. A ‘slide’ score was then calculated for the subject between 0 and 1 based on the number of actions performed. A score of 1 represented 20 slides (100% slides) whereas a score of 0 represented 20 lifts). In the single case of no food retrieval, and therefore no method bias, a score of 0.5 was given. A two-tailed Mann–Whitney test showed that the two chains initiated with either lift or slide methods (i.e. excluding the initial models) were significantly different in their slide score (median lift chain=0, median slide chain=1; Z=−2.61, n1=5, n2=4, p=0.01)
The first four lift-group observers performed 100% lift while the last subject in the chain performed 90% lift (figure 3b, with slide actions at trials 15 and 17 in a total of 20 trials). The first three slide-group subjects performed 100% slide, while the last subject in the chain performed 95% slide (figure 3c), with one lift at trial 7, in a total of 20 trials. Although some corruption emerged for the last monkey in each chain, these were isolated incidents followed by responses that continued to replicate the actions of the prior monkey. It should be emphasized that chains were not terminated at this point owing to these results, but because these were the maximum number of subjects available and assigned to the experimental design.
During the observer testing phase, only one subject in each test group (RO and AL) did not immediately open the apparatus; instead, they spent 8 and 24 s, respectively, feeling the front panel before acting on the door. During the control testing phase, the unsuccessful subject (KO) manipulated the Doorian, touching the handle, door and various other parts of the device several times throughout the 15 min, but never opened the door.
4. Discussion
This study demonstrates that capuchin monkeys are capable of learning a foraging technique from a conspecific demonstrator and that this process will repeat over several cultural generations of group members. To our knowledge, this kind of finding has not previously been shown experimentally in monkeys, and adds to a small body of experiments demonstrating socially learned diffusion effects in a variety of vertebrates (Curio et al. 1978; Lefebvre 1986; Laland & Plotkin 1990; Laland & Williams 1997; Reader & Laland 2000). However, these earlier studies contrasted only a single experimental group with controls, and thus concern only a single behaviour pattern such as pecking through a paper cover to gain food (Lefebvre 1986). In such experimental designs, effects may reflect only the facilitation or targeting of existing elements of behaviour. For example, if we had used only a slide model compared with non-observing controls, a greater occurrence of slide in the first group might be because they had discovered through observation that food was in the box and slide came naturally to them as a means to obtain it, while controls remained ignorant of this opportunity. By contrast, the two-action aspect of our design shows, crucially, that some kind of copying process was at work, to provide the necessary differentiation between the replications that occurred along each chain of individuals seeded with the alternative methods.
To our knowledge, ours is the first two-action transmission chain study to demonstrate such an effect in monkeys and indeed in any non-human species other than the chimpanzees studied by Horner et al. (2006). Moreover, the tendency of the two individuals who discovered (possibly by accident) the alternative method to nevertheless stay faithful to the method, they had observed hints at the kind of conformity to group-mates' methods described in recent chimpanzee experiments (Whiten et al. 2005). Because this concerns only two individuals, this must remain a tentative interpretation at this point, but deserves more attention in future studies. Nevertheless, the fidelity of transmission we documented remains remarkable given the potential for corruption, and since one might expect that a monkey attempting to lift could all too easily accidentally discover slide, or vice versa (in both cases its hand is on the same handle).
Our study can draw limited conclusions about the social learning mechanism (or mechanisms) at work and was not designed to do so, other than controlling for processes as elementary as stimulus enhancement, by ensuring that the same handle was used to open the door by either lifting or sliding. Ruling out stimulus enhancement means that more sophisticated processes are implicated, with some capacity for copying of either actions (lift versus slide—‘imitation’), or the results of such actions (door rising versus door sliding—‘emulation’: Tomasello & Call 1997). That three of the four controls were able to solve the task by either lifting or sliding suggests that these basic capacities were available to all subjects but channelled into one form or the other by social learning. In any case, further experiments will be needed to discriminate these, such as ‘ghost’ conditions in which observers see only the door move (Tennie et al. 2006; Hopper et al. 2007). Given that previous studies with capuchin monkeys have shown little evidence for imitation, we provided the capuchins with relatively straightforward tasks, which we anticipated might be easily assimilated by them (as well as easily discriminable by the experimenter coding the tests). Further research could expand upon this to investigate more complex manipulations or sequential tasks in order to gain further insight into capuchins' copying abilities.
Whatever the precise mechanism, our two-action transmission chain study has demonstrated a capacity in capuchin monkeys for serial transmission of alternative behaviour patterns. Why we recorded so much greater copying fidelity than the majority of earlier studies with capuchins and other monkeys is not known, but we suspect at least two factors may have been important. First, we took great care to modify the task in a number of respects (see §2) so that it was well suited to the behavioural capacities of the study species; and second, we took great care to perform compatibility checks for each pair of individuals in the experimental chains. The latter may raise an alternative concern that we engineered greater tolerance than would exist for natural opportunities for cultural transmission in this species. Although the generally tolerant nature of capuchins (Ottoni et al. 2005) would appear to make this unlikely, it would be beneficial to supplement our diffusion chain study with one based on the freedom of open diffusion to further examine the role of dominance.
Our study was restricted by subject availability to chains of the lengths achieved, so stands in need of further replication and, ideally, extension to longer chains as well as more naturalistic open diffusion experiments in which whole groups are exposed to expert models (Bonnie et al. 2007). Nevertheless, the transmission effects we documented are statistically robust. They are consistent with field ethologists' interpretations of their observational data, which suggest that capuchins in the wild sustain socially transmitted traditions.
Acknowledgments
This project was conducted at the Centre de Primatologie in Strasbourg, France, under CNRS guidelines and in compliance with all French legal and ethical requirements for research with primates. This research adhered to the Association for the Study of Animal Behaviour/Animal Behaviour Society Guidelines for the Use of Animals in Research (published on the Animal Behaviour website), the legal requirements of the country in which the work was carried out and all institutional guidelines. Support for this project was provided by the BBSRC (A.W.), the Leverhulme Trust (A.W.), the Royal Society (A.W.) and CNRS (B.T.). We are grateful to Andy Burnley for constructing the Doorian apparatus, to Valerie Dufour, Odile Petit and Pierre H. Ulrich for their help involving the planning and management of the project and to Kristin Bonnie, Frans de Waal and two referees for their helpful comments on the manuscript.
Supplementary Material
A model demonstrates the lift method for extracting food rewards, while the observer watches
The observer subject's actions after watching a lift model
A model demonstrates the slide method for extracting food rewards, while the observer watches
The observer subject's actions after watching a slide model
References
- Adams-Curtis L, Fragaszy D.M. Influence of a skilled model on the behavior of conspecific observers in tufted capuchin monkeys (Cebus apella) Am. J. Primatol. 1995;37:65–71. doi: 10.1002/ajp.1350370107. doi:10.1002/ajp.1350370107 [DOI] [PubMed] [Google Scholar]
- Aisner R, Terkel J. Ontogeny of pinecone opening behaviour in the black rat, Rattus rattus. Anim. Behav. 1992;44:327–336. doi:10.1016/0003-3472(92)90038-B [Google Scholar]
- Bartlett F. Macmillan; Oxford, UK: 1932. Remembering. [Google Scholar]
- Bonnie K.E, de Waal F.B.M. Copying without rewards: socially influenced foraging decisions among brown capuchin monkeys. Anim. Cogn. 2007;10:283–292. doi: 10.1007/s10071-006-0069-9. doi:10.1007/s10071-006-0069-9 [DOI] [PubMed] [Google Scholar]
- Bonnie K, Horner V, Whiten A, de Waal F. Spread of arbitrary conventions among chimpanzees: a controlled experiment. Proc. R. Soc. B. 2007;274:367–372. doi: 10.1098/rspb.2006.3733. doi:10.1098/rspb.2006.3733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd R, Richerson P.J. University of Chicago Press; Chicago, IL: 1985. Culture and the evolutionary process. [Google Scholar]
- Brown C, Laland K.N. Social learning in fishes. In: Brown C, Laland K.N, Krause J, editors. Fish cognition and behavior. Blackwell; Oxford, UK: 2006. pp. 186–202. [Google Scholar]
- Coussi-Korbel S, Fragaszy D.M. On the relation between social dynamics and social learning. Anim. Behav. 1995;50:1441–1453. doi:10.1016/0003-3472(95)80001-8 [Google Scholar]
- Curio E, Ernst U, Vieth W. The adaptive significance of avian mobbing: II. Cultural transmission of enemy recognition in blackbirds: effectiveness and some constraints. Z. Tierpsych. 1978;48:184–202. [Google Scholar]
- Danchin E, Giraldeau L.A, Valone T.J, Wagner R.H. Public information: from noisy neighbors to cultural evolution. Science. 2004;305:487–491. doi: 10.1126/science.1098254. doi:10.1126/science.1098254 [DOI] [PubMed] [Google Scholar]
- Dawson B.V, Foss B.M. Observational learning in budgerigars. Anim. Behav. 1965;13:470–474. doi: 10.1016/0003-3472(65)90108-9. doi:10.1016/0003-3472(65)90108-9 [DOI] [PubMed] [Google Scholar]
- Fragaszy D. Making space for traditions. Evol. Anthropol. 2003;12:61–70. doi:10.1002/evan.10104 [Google Scholar]
- Fragaszy D.M, Perry S. Cambridge University Press; Cambridge, UK; New York, NY: 2003. The biology of traditions: models and evidence. [Google Scholar]
- Fragaszy D.M, Visalberghi E. Recognizing a swan: socially-biased learning. Psychologia. 2001;44:82–98. [Google Scholar]
- Fragaszy D.M, Visalberghi E. Socially biased learning in monkeys. Learn. Behav. 2004;32:24–35. doi: 10.3758/bf03196004. [DOI] [PubMed] [Google Scholar]
- Fredman, T. & Whiten, A. In press. Observational learning from tool using models in human-reared and mother-reared capuchin monkeys (Cebus apella). Anim. Cog [DOI] [PubMed]
- Galef B.G. “Traditional” foraging behaviours of brown and black rats (Rattus norvegicus and Rattus rattus) In: Fragaszy D.M, Perry S, editors. The biology of traditions: models and evidence. Cambridge University Press; Cambridge, UK: 2003. pp. 159–186. [Google Scholar]
- Galef B.G, Giraldeau L.A. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 2001;61:3–15. doi: 10.1006/anbe.2000.1557. doi:10.1006/anbe.2000.1557 [DOI] [PubMed] [Google Scholar]
- Galef B.G, Heyes C.H.E. Special issue on social learning in animals. Learn. Behav. 2004;32:1–140. [Google Scholar]
- Galef B.G, Manzig L.A, Field R.M. Imitation learning in budgerigars—Dawson and Foss (1965) revisited. Behav. Process. 1986;13:191–202. doi: 10.1016/0376-6357(86)90025-2. doi:10.1016/0376-6357(86)90025-2 [DOI] [PubMed] [Google Scholar]
- Goodall J. Cultural elements in a chimpanzee community. In: Menzel E.W.J, editor. Precultural primate behaviour. Karger; Basel, Germany: 1973. pp. 144–184. [Google Scholar]
- Hopper L.M, Spiteri A, Lambeth S.P, Schapiro S.J, Horner V, Whiten A. Experimental studies of traditions and underlying transmission processes in chimpanzees. Anim. Behav. 2007;73:1021–1032. doi:10.1016/j.anbehav.2006.07.016 [Google Scholar]
- Horner V, Whiten A, Flynn E, de Waal F.B.M. Faithful replication of foraging techniques along cultural transmission chains by chimpanzees and children. Proc. Natl Acad. Sci. USA. 2006;103:13 878–13 883. doi: 10.1073/pnas.0606015103. doi:10.1073/pnas.0606015103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley S, Chater N. MIT Press; Cambridge, MA: 2005. Perspectives on imitation: from neuroscience to social science. [Google Scholar]
- Kendal R.L, Coe R.L, Laland K.N. Age differences in neophilia, exploration, and innovation in family groups of Callitrichid monkeys. Am. J. Primatol. 2005;66:167–188. doi: 10.1002/ajp.20136. doi:10.1002/ajp.20136 [DOI] [PubMed] [Google Scholar]
- Laland K.N, Hoppitt W. Do animals have culture? Evol. Anthropol. 2003;12:150–159. doi:10.1002/evan.10111 [Google Scholar]
- Laland K.N, Plotkin H.C. Social-learning and social transmission of foraging information in Norway rats (Rattus norvegicus) Anim. Learn. Behav. 1990;18:246–251. [Google Scholar]
- Laland K.N, Plotkin H.C. Further experimental analysis of the social learning and transmission of foraging information amongst Norway rats. Behav. Proc. 1992;27:53–64. doi: 10.1016/0376-6357(92)90040-K. doi:10.1016/0376-6357(92)90040-K [DOI] [PubMed] [Google Scholar]
- Laland K.N, Williams K. Shoaling generates social learning of foraging information in guppies. Anim. Behav. 1997;53:1161–1169. doi: 10.1006/anbe.1996.0318. doi:10.1006/anbe.1996.0318 [DOI] [PubMed] [Google Scholar]
- Laland K.N, Williams K. Social transmission of maladaptive information in the guppy. Behav. Ecol. 1998;9:493–499. doi:10.1093/beheco/9.5.493 [Google Scholar]
- Leca J.B, Gunst N, Huffman M.A. Japanese macaque cultures: inter- and intra-troop behavioural variability of stone handling patterns across 10 troops. Behaviour. 2007;144:251–281. doi:10.1163/156853907780425712 [Google Scholar]
- Lefebvre L. Cultural diffusion of a novel food-finding behaviour in urban pigeons: an experimental field test. Ethology. 1986;71:295–304. [Google Scholar]
- Nishida T, Wrangham R.W, Goodall J, Uehara S. Local differences in plant-feeding habits of chimpanzees between the Mahale Mountains and Gombe National Park, Tanzania. J. Hum. Evol. 1983;12:467–480. doi:10.1016/S0047-2484(83)80142-0 [Google Scholar]
- Ottoni E.B, Mannu M. Semifree-ranging tufted capuchins (Cebus apella) spontaneously use tools to crack open nuts. Int. J. Primatol. 2001;22:347–358. doi:10.1023/A:1010747426841 [Google Scholar]
- Ottoni E.B, de Resende B.D, Izar P. Watching the best nutcrackers: what capuchin monkeys (Cebus apella) know about others' tool-using skills. Anim. Cogn. 2005;8:215–219. doi: 10.1007/s10071-004-0245-8. doi:10.1007/s10071-004-0245-8 [DOI] [PubMed] [Google Scholar]
- Panger M.A, Perry S, Rose L, Gros-Louis J, Vogel E, Mackinnon K.C, Baker M. Cross-site differences in foraging behavior of white-faced capuchins (Cebus capucinus) Am. J. Phys. Anthropol. 2002;119:52–66. doi: 10.1002/ajpa.10103. doi:10.1002/ajpa.10103 [DOI] [PubMed] [Google Scholar]
- Perry S.E. What cultural primatology can tell anthropologists about the evolution of culture. Annu. Rev. Anthropol. 2006;35:171–190. doi:10.1146/annurev.anthro.35.081705.123312 [Google Scholar]
- Perry S, et al. Social conventions in wild white-faced capuchin monkeys—evidence for traditions in a neotropical primate. Curr. Anthropol. 2003;44:241–268. doi:10.1086/345825 [Google Scholar]
- Reader S.M, Laland K.N. Diffusion of foraging innovations in the guppy. Anim. Behav. 2000;60:175–180. doi: 10.1006/anbe.2000.1450. doi:10.1006/anbe.2000.1450 [DOI] [PubMed] [Google Scholar]
- Shettleworth S.J. Animal cognition and animal behaviour. Anim. Behav. 2001;61:277–286. doi:10.1006/anbe.2000.1606 [Google Scholar]
- Stanley, E. L., Kendal, R. L., Kendal, J. R., Grounds, S. & Laland, K. N. In press. The effects of group size, rate of turnover and disruption to demonstration on the stability of foraging traditions in fishes. Anim. Behav (doi:10.1016/j.anbehav.2007.06.014)
- Tennie C, Call J, Tomasello M. Push or pull: emulation versus imitation in great apes and human children. Ethology. 2006;112:1159–1169. doi:10.1111/j.1439-0310.2006.01269.x [Google Scholar]
- Terkel J. Cultural transmission of feeding behaviour in the black rat (Rattus rattus) In: Heyes C.M, Galef B.G Jr, editors. Social learning in animals: the roots of culture. Academic Press; London, UK: 1996. pp. 17–48. [Google Scholar]
- Tomasello M, Call J. Oxford University Press; New York, NY: 1997. Primate cognition. [Google Scholar]
- van Schaik C.P, Ancrenaz M, Borgen G, Galdikas B, Knott C.D, Singletin I, Suzuki A, Utami S.S, Merrill M. Orangutan cultures and the evolution of material culture. Science. 2003;299:102–105. doi: 10.1126/science.1078004. doi:10.1126/science.1078004 [DOI] [PubMed] [Google Scholar]
- Visalberghi E, Fragaszy D.M. Do monkeys ape? In: Parker T.S, Gibson K.R, editors. “Language” and intelligence in monkeys and apes: comparative developmental perspectives. Cambridge University Press; Cambridge, UK: 1990. pp. 247–273. [Google Scholar]
- Visalberghi E, Fragaszy D.M. Do monkeys ape? Ten years after. In: Dautenhahn K, Nehaniv C.L, editors. Imitation in animals and artifacts. MIT Press; Cambridge, MA: 2002. pp. 471–500. [Google Scholar]
- Whiten A. The second inheritance system of chimpanzees and humans. Nature. 2005;437:52–55. doi: 10.1038/nature04023. doi:10.1038/nature04023 [DOI] [PubMed] [Google Scholar]
- Whiten A, van Schaik C.P. The evolution of animal ‘cultures’ and social intelligence. Phil. Trans. R. Soc. B. 2007;362:603–620. doi: 10.1098/rstb.2006.1998. doi:10.1098/rstb.2006.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiten A, Goodall J, McGrew W.C, Nishida T, Reynolds V, Sugiyama Y, Tutin C.E.G, Wrangham R.W, Boesch C. Cultures in chimpanzees. Nature. 1999;399:682–685. doi: 10.1038/21415. doi:10.1038/21415 [DOI] [PubMed] [Google Scholar]
- Whiten A, Horner V, de Waal F.B.M. Conformity to cultural norms of tool use in chimpanzees. Nature. 2005;437:737–740. doi: 10.1038/nature04047. doi:10.1038/nature04047 [DOI] [PubMed] [Google Scholar]
- Whiten A, Spiteri A, Horner V, Bonnie K.E, Lambeth S.P, Schapiro S.J, de Waal F.B.M. Transmission of multiple traditions within and between chimpanzee groups. Curr. Biol. 2007;17:1038–1043. doi: 10.1016/j.cub.2007.05.031. doi:10.1016/j.cub.2007.05.031 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A model demonstrates the lift method for extracting food rewards, while the observer watches
The observer subject's actions after watching a lift model
A model demonstrates the slide method for extracting food rewards, while the observer watches
The observer subject's actions after watching a slide model