Abstract
Methods to determine cytokine protein content in samples of interest, such as Enzyme- Linked ImmunoSorbent Assay (ELISA), are often labor-intensive and costly. Furthermore, because ELISA requires relatively large sample volumes and protein concentrations, it is difficult using this technique to determine protein content for multiple cytokines from individual samples. Recently, Luminex® has developed an open source hardware platform combining flow cytometry and bead-based antibody capture that is capable of detecting multiple analytes from a single sample. In the present study we employed the Luminex 200® platform, to determine the cytokine protein content in discrete brain regions of C57BL/6J mice. In spike-and-recovery experiments, known concentrations of murine recombinant interleukin (IL)-1β, IL-6, and tumor necrosis factor (TNF)α were added either singly or as a mixture of all three to whole brain homogenates containing known quantities of total protein. Spiked samples were assayed for either a single cytokine or for multiple cytokines using 1-plex or 3-plex assay kits, respectively. In whole mouse brain homogenate we recovered between 81% to 103% of the recombinant cytokines. We then injected C57BL/6J mice intraperitoneally with bacterial lipopolysaccharide (LPS) and sacrificed them 4 h later. We detected in samples taken from LPS-stimulated mice 4 to 870 fold increases in serum or spleen cytokine protein, and 1.5 to 16 fold increases in cytokine protein in discrete brain regions, relative to protein content in samples obtained from vehicle-treated animals. These results indicate that multiple cytokines may be reliably assayed from discrete regions of mouse brain using a single sample.
Keywords: sleep, mouse, multiplex, interleukin, tumor necrosis factor, brain, CNS
INTRODUCTION
Cytokines are immune regulators that were historically thought to be products solely of the peripheral immune system. It is now well established that several cytokines and their receptors are present in normal brain, where they are involved in the regulation of multiple physiological processes and complex behavior [e.g.(Gibertini, 1998;Turnbull and Rivier, 1999;Leon, 2002;Opp, 2005;Dantzer, 2006)]. Examples of central nervous system (CNS) processes and behavior in which cytokines have been implicated include, among others, sleep, temperature regulation, locomotor activity, sexual behavior, memory and cognition and feeding behavior. Because cytokines and their receptors are present in normal, healthy brain, cytokine systems are well-positioned to mediate alterations in physiology and behavior that occur during the course of immune challenge. For example, cytokines have been implicated in the alterations in body temperature and sleep that are apparent in laboratory animals when infected with a variety of pathogens [reviewed (Toth and Opp, 2002)]. For these, and other reasons, the assessment of cytokine proteins in brain is now important in neurobiology, particularly with respect to pathologies associated with inflammatory processes.
A number of methods have been used to quantify cytokine protein content in tissues and cells. These methodologies include, but are not limited to enzyme-linked immunosorbent assay (ELISA), protein microarrays, radio immunoassay (RIA), bioassays and multi-parametric flow cytometry. ELISA is generally considered the gold standard for protein quantitation, and there are several advantages to the use of this methodology. However, there are also some limitations. For example, ELISA is limited to the analysis of one analyte at a time. Although some have used sequential ELISAs to quantify multiple cytokines from a single sample (O'connor, Holguin et al., 2004;Osuchowski, Siddiqui et al., 2005), a separate ELISA must be used for each analyte of interest. In addition, the necessity of running multiple ELISAs to determine protein concentrations of different cytokines becomes labor intensive, and often cost prohibitive, as the number of analytes of interest increases.
Once the animal of choice primarily for immunologists, the mouse is now increasingly used in the field of neuroscience due to the relative ease with which its genome may be manipulated. One of the limitations of using mice in neurobiology, however is the relatively small size of the brain. The small brain size results in small starting amounts of material if assessment of protein is a desired outcome measure. In addition, because the brain is not a homogeneous structure, it is necessary to be able to quantify protein content in discrete brain regions, which further limits the starting material available for assay. Finally, regulatory proteins in brain are generally of low abundance, which requires sensitive methods for protein detection. As such, methods that allow determination of multiple proteins from a single sample would be useful for mouse neurobiology. The recent development of xMAP® technology based on the Luminex platform (Luminex Corporation, Austin, TX USA) holds promise as a method by which multiple cytokines may be quantified from single samples of small volume obtained from discrete mouse brain regions. We present in this validation study results of spike-recovery experiments, and quantification of changes in cytokine protein in mouse brain regions following peripheral immune challenge with bacterial lipopolysaccharide.
MATERIALS AND METHODS
Substances
Recombinant murine interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)-α were purchased from R&D Systems, Inc. (Minneapolis, MN). The cytokines were provided as lyophilized powder, and were reconstituted in phosphate-buffered saline (pH 7.4) containing 0.5% bovine serum albumin and 0.02% sodium azide. Volumes of the stock concentration (20 µg/ml) were aliquoted and stored at −20 °C until use. A new aliquot was used for each assay to avoid repeated freeze-thaw cycles.
Animals
Adult male C57BL/6J mice were purchased from Jackson Laboratory (Bar Harbor, ME). These animals were housed on a 12:12h light:dark cycle at 29 ± 1 °C with food and water available ad libitum. For studies in which recovery of cytokines from whole brain homogenates was determined, two undisturbed mice were sacrificed 4-h after light onset. The entire brain from each mouse was quickly removed, placed in a cryovial and snap frozen in liquid nitrogen until assay. The homogenate from one brain was used for determining recovery from 1-plex kits, whereas homogenate from the other brain was used to determine recovery from 3-plex kits (see later and Table 1, Table 2).
Table 1.
Determination of cytokine protein from C57BL/6J mouse brain homogenate spiked with known quantities of murine recombinant interleukin-1β (IL-1β), IL-6, or tumor necrosis factor α (TNFα). Assays for each cytokine were run separately using 1-plex kits. Cytokine protein detected in unspiked samples of whole brain homogenate represent basal cytokine expression in C57BL/6J mouse brain during this portion of the light:dark cycle. All samples were run in duplicate.
| Protein in whole brain homogenate | Amount of recombinant cytokine added | Recombinant IL-1β detected (% of spike) | Recombinant IL-6 detected (% of spike) | Recombinant TNFα detected (% of spike) |
|---|---|---|---|---|
| 0 µg | 0 pg | ND | ND | ND |
| 5 pg | 4 pg (80) | 4 pg (80) | 4 pg (80) | |
| 20 pg | 18 pg (90) | 18 pg (90) | 19 pg (95) | |
| 50 pg | 46 pg (92) | 47 pg (94) | 45 pg (90) | |
| 500 pg | 478 pg (96) | 485 pg (97) | 467 pg (93) | |
| 100 µg | 0 pg | 15 pg | 29 pg | 26 pg |
| 5 pg | 18 pg (90) | 33 pg (97) | 29 pg (94) | |
| 20 pg | 28 pg (80) | 47 pg (96) | 40 pg (87) | |
| 50 pg | 54 pg (83) | 75 pg (95) | 68 pg (89) | |
| 500 pg | 503 pg (98) | 513 pg (97) | 482 pg (92) | |
| 200 µg | 0 pg | 33 pg | 64 pg | 52 pg |
| 5 pg | 36 pg (95) | 68 pg (99) | 54 pg (95) | |
| 20 pg | 46 pg (87) | 82 pg (98) | 66 pg (92) | |
| 50 pg | 78 pg (89) | 110 pg (96) | 96 pg (94) | |
| 500 pg | 504 pg (95) | 525 pg (93) | 478 pg (87) |
ND: not detected.
Table 2.
Determination of cytokine protein from C57BL/6J mouse brain homogenate spiked with known quantities of murine recombinant interleukin-1β (IL-1β), IL-6, or tumor necrosis factor α (TNFα). Three-plex assays were run to determine cytokine protein concentrations simultaneously. Cytokine protein detected in unspiked samples of whole brain homogenate represent basal cytokine expression in C57BL/6J mouse brain during this portion of the light:dark cycle. All samples were run in duplicate.
| Protein in whole brain homogenate | Amount of recombinant cytokine added (IL-1β/IL-6/TNFα) | Recombinant IL-1β detected (% of spike) | Recombinant IL-6 detected (% of spike) | Recombinant TNFα detected (% of spike) |
|---|---|---|---|---|
| 0 µg | 0/0/0/ pg | ND | ND | ND |
| 5/5/5 pg | 4 pg (80) | 4 pg (80) | 5 pg (100) | |
| 20/20/20 pg | 19 pg (95) | 19 pg (95) | 19 pg (95) | |
| 50/50/50 pg | 46 pg (92) | 44 pg (88) | 46 pg (92) | |
| 500/500/500 pg | 484 pg (97) | 499 pg (100) | 493 pg (99) | |
| 100 µg | 0/0/0/ pg | 14 pg | 27 pg | 22 pg |
| 5/5/5 pg | 17 pg (89) | 30 pg (94) | 25 pg (93) | |
| 20/20/20 pg | 32 pg (94) | 45 pg (96) | 37 pg (88) | |
| 50/50/50 pg | 59 pg (92) | 70 pg (91) | 65 pg (90) | |
| 500/500/500 pg | 482 pg (94) | 497 pg (94) | 464 pg (93) | |
| 200 µg | 0/0/0/ pg | 26 pg | 50 pg | 41 pg |
| 5/5/5 pg | 32 pg (103) | 54 pg (98) | 44 pg (96) | |
| 20/20/20 pg | 46 pg (100) | 67 pg (96) | 56 pg (92) | |
| 50/50/50 pg | 71 pg (93) | 87 pg (87) | 80 pg (88) | |
| 500/500/500 pg | 484 pg (92) | 514 pg (93) | 484 pg (89) |
ND: not detected.
For determination of the impact of immune challenge on cytokine protein in mouse brain, separate mice were injected intraperitoneally at light onset with either 0.2 ml vehicle (pyrogen-free saline; n = 4) or with 10 µg lipopolysaccharide (LPS; Escherichia coli serotype O111:B4; Sigma-Aldrich, St. Louis, MO; n = 4). These mice were sacrificed 4 h later and trunk blood collected and the spleen removed. The brains from these animals were quickly removed, placed on an ice-cold surface, and the hypothalamus, hippocampus, and brainstem (pons) were dissected. All tissues were snap-frozen in liquid nitrogen and stored at −80 °C until processing. The blood was centrifuged (12,000 × g, 4 °C, 20 min) and serum stored at −80 °C until assay. All procedures involving the use of animals were approved by the University Committee on Care and Use of Animals in accordance with all federal regulations.
Protein extractions
Frozen tissues were thawed and disrupted in Bioplex cell lysis buffer (BioRad catalog # 171-304011) containing factors 1 and 2 (protease and phosphatase inhibitors, respectively; BioRad catalog #171-304012) and the protease inhibitor phenyl-methylsulfonyl fluoride (PMSF, 500 mM; Sigma-Aldrich). Tissue was disrupted by drawing samples up and down through either 200 µl or 500 µl pipette tips from which the ends had been cut to make a larger opening. After repeated drawing in-and-out of the pipette, a small pellet pestle (Kontes, Vineland, NJ) was used to homogenize the sample. The homogenate was then agitated for 30 – 40 min on ice (Rocky Platform model 100; VWR Scientific, West Chester, PA) and centrifuged at 4 °C and 6,000 × g (Sorvall Biofuge Fresco) for 20 min. The supernatant was removed and aliquoted. Aliquots were stored at −80 °C until assay. The protein content of each sample was determined using the bicinchoninic acid (BCA) assay (Pierce catalog # 23225, Rockford, IL), with bovine serum albumin (BSA) as a standard, according to the manufacturer’s protocol. Sample absorbances were read at 560 nm using a spectrophotometer (Molecular Devices Versa Max, Sunnyvale, CA).
Sample preparation
Stock aliquots of recombinant murine IL-1β, IL-6 and TNF-α were thawed immediately prior to assay. As such, recombinant cytokines used in the recovery experiments were subjected to one freeze-thaw cycle. Working solutions ranging in concentration from 1 – 500 pg/µl were made by diluting the stock concentration in cell lysis buffer. For recovery determinations of 1-plex assays, concentrations of 0, 5, 20, or 500 pg (in 10 µl cell lysis buffer) of the individual cytokines were added to either 100 µg or 200 µg protein (in 40 µl cell lysis buffer) from whole mouse brain homogenates. Therefore, the total volume of each sample was 50 µl. For 3-plex assays, cytokine concentrations of 0, 5, 20, or 500 pg were prepared in 1 µl of cell lysis buffer. These cytokines were mixed in equal concentrations and equal volumes (1 µl for each cytokine) with either 100 µg or 200 µg (in 40 µl cell lysis buffer) and the total volume of each sample was brought to 50 µl with the addition of 7 µl cell lysis buffer.
Serum samples were diluted 1:3 with serum sample diluent (BioRad catalog #171-305-008) prior to assay. All samples (serum and protein samples from tissue) were held at 4 °C for 10 min before the start of the assay. Corresponding buffer blanks were run to allow determination of any nonspecific background readings.
Determination of cytokine concentrations
BioRad (Hercules, CA) Bio-Plex kits were used for all assays. All samples were run in duplicate and were assayed for murine IL-1β, IL-6, and TNF-α using single-plex (BioRad catalog #: IL-1β, 171-G12819; IL-6, 171-G10738; TNF-α, 171-G12221) or multi-plex (IL-1β , IL-6, TNF-α # X6000000X1) bead-based kits, the cytokine reagent kit (Cat. # 171-304000) and either the diluent kit for serum samples (Cat. # 171-305004) or cell lysis kit for tissue samples (Cat. # 171-304011). The assays were run according to manufactures recommended procedures. In brief, all buffers and diluents were warmed to room temperature prior to use. Lyophilized cytokine standards were reconstituted first to a master standard stock using 500 µl diluent according to manufacturer’s directions. Four-fold serial dilutions of the master standard stock provided eight concentrations used to determine a standard curve. The concentration ranges of the standards were: IL-6: 0.14 – 2312 pg/ml; TNFα: 0.34 – 5594 pg/ml; IL-1β: 0.22 – 1721 pg/ml.
The assays were run according to instructions included with the Bio-Plex kits. General practices included keeping reagents on ice until use, minimizing exposure of the beads to light by wrapping tubes and/or plates with aluminum foil, and using appropriate agitation on a microplate shaker (initial agitation at 1100 rpm for 1 min and then reduced agitation at 300 rpm for 30 min).
After completion of all steps in the assay, the plates were read in the Bio-Plex 200 System and the data analyzed using BioPlex Manager 4.1 software with five parameter logistic regression (5PL) curve fitting. The goodness of fit for each point on the standard curve was determined by the BioPlex Manager software as a back-calculation of standards. The back-calculation was made with the following equation:
Each point on the standard curve was within the acceptable range of 70 – 130% of actual levels, which is an acceptable range for this measure (Nix and Wild, 2001).
Values from tissue or serum samples that had not been spiked with recombinant cytokines were used to determine basal (endogenous) cytokine concentrations. Any increases in cytokine concentrations in samples from mice after stimulation / manipulation are superimposed upon basal amounts of cytokines already present. Furthermore, assays of cytokines obtained from tissue homogenates or serum by this method provide quantification of the total amount of cytokine present in the sample without regard for the source of the protein. Therefore, our calculations of cytokine recovery accounted for the endogenous cytokines normally present in brain. We calculated the percent recovery for each spiked sample using the following equation:
For example, if 15 pg/ml of a particular cytokine was detected in a sample of whole brain homogenate that had not been spiked (0 cytokine added) and 18 pg/ml of that cytokine was detected in a sample of that brain homogenate that had been spiked with 5 pg/ml of recombinant cytokine, we would state that 90 % of the spiked sample had been recovered: [(18 pg/ml observed)/(15 pg ml basal + 5 pg ml added)] * 100. This formula thus yields a value that indicates the “percent of total” protein recovered.
Statistics
Statistical analyses were conducted using SPSS for Windows (SPSS, Inc., Chicago, IL). Pearson’s correlation coefficients were used to determine correspondence between standard curves for each cytokine obtained from 1-plex and from 3-plex kits. Comparisons of values for cytokine protein content in unstimulated whole brain homogenates (i.e., basal concentrations) detected using 1-plex kits and 3-plex kits were made using t-tests for independent samples. In these analyses, the grouping variable was kit (1-plex vs. 3-plex) while the test variables were the concentrations of the three cytokines (IL-1β, IL-6, TNFα). Changes in cytokine concentrations in discrete brain regions induced by LPS were also statistically evaluated using t-tests for independent samples. In this case, manipulation (vehicle vs. LPS) was the grouping variable. Comparisons were made within the same sample type (ie., serum vs. serum, spleen vs. spleen, etc.). An alpha level of p ≤ 0.05 was accepted as indicating significant differences between groups.
RESULTS
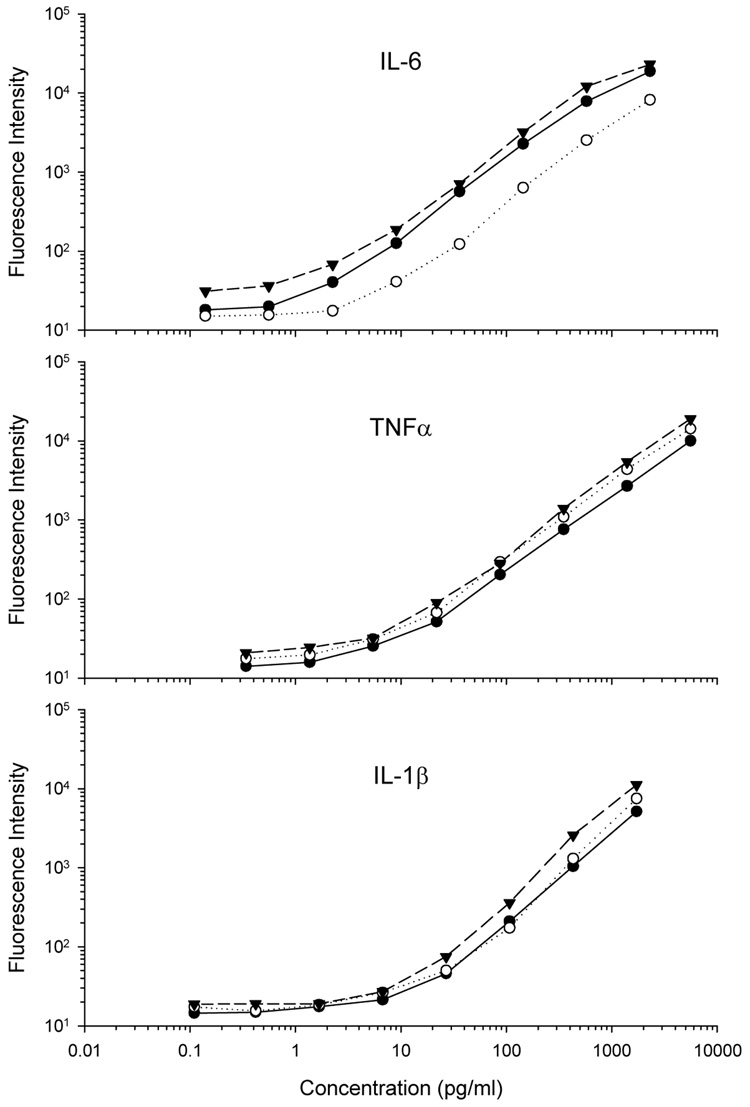
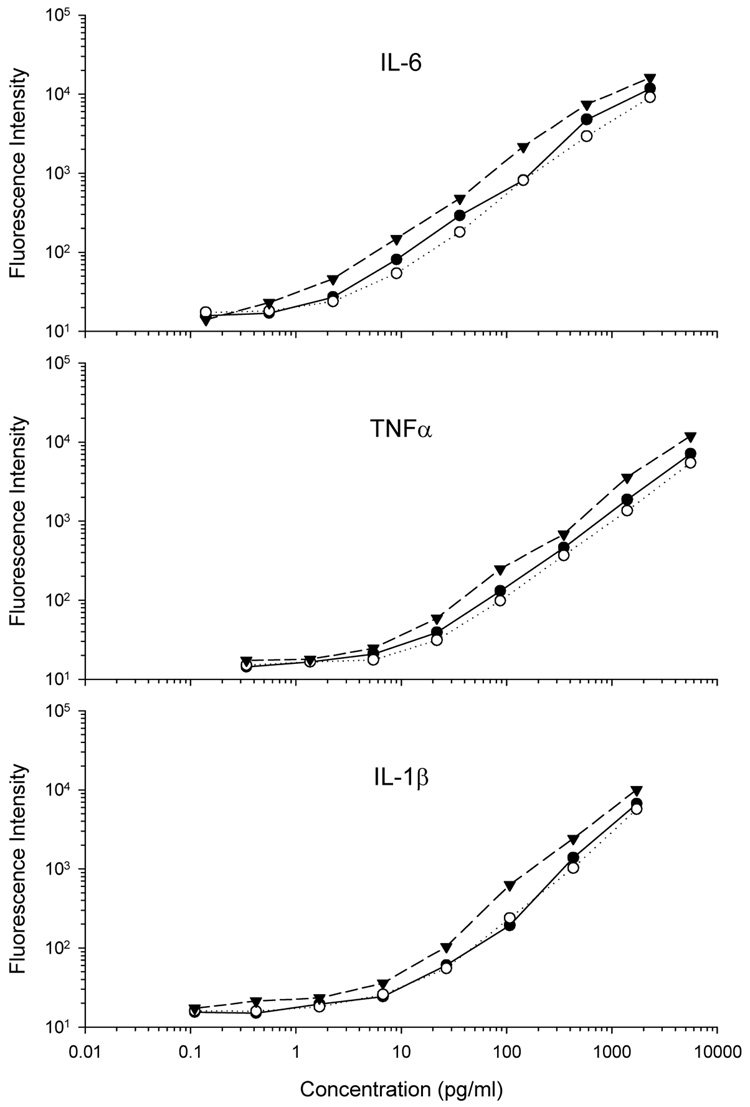
A total of 14 Bio-Plex kits were used in this study. Nine 1-plex kits were used to assay all single-analyte samples (Figure 1, Table 1), whereas three 3-plex kits were used for determining multiple cytokines from the same sample in the spike-and-recovery experiments (Figure 2, Table 2). Two additional 3-plex kits were used to determine cytokine concentrations in samples obtained from mice after LPS challenge (Table 3). Depending on the batch of beads, the fluorescent intensity between kits occasionally varied (e.g., Figure 1, IL-6). However, the ratio of protein concentration specified by the manufacturer (actual) to the concentration of protein determined as part of the standard curve (observed) was always within accepted limits, irrespective of the fluorescent intensity. According to the manufacturers specifications, the dynamic range for these kits is approximately 4 – 5 log units. Correlational analyses indicated very good correspondence in cytokine protein concentrations between standard curves obtained from 1-plex and 3-plex kits (Figure 1, Figure 2). Pearson’s correlation coefficients for standards were 0.999 for IL-6, 1.000 for IL-1β, and 0.998 for TNFα.
Figure 1.
Standard curves for nine 1-plex BioPlex (BioRad, Hercules, CA) kits. Each kit was used to determine concentrations of interleukin-1β (IL-1β), IL-6 or tumor necrosis factor α (TNFα) from separate assays. Standards were prepared according to manufacturer’s instructions, and all standards were run in duplicate. Readings were obtained using BioPlex 200 hardware running BioPlex manager software (v. 4.1). A five parameter logistic regression model (5PL) was used to fit the curve.
Figure 2.
Standard curves for three 3-plex BioPlex (BioRad, Hercules, CA) kits. Each kit was used to determine concentrations of interleukin (IL)-1β, IL-6 and tumor necrosis factor (TNF)α simultaneously. Standards were prepared according to manufacturer’s instructions, and all standards were run in duplicate. Readings were obtained using BioPlex 200 hardware running BioPlex manager software (v. 4.1). A five parameter logistic regression model (5PL) was used to fit the curve.
Table 3.
Effects of intraperitoneal injection of lipopolysaccharide (LPS) on cytokine concentrations in samples obtained from C57BL/6J mice. Mice were injected with LPS at light onset and sacrificed 4 hours later. Cytokine concentrations from 50 µg total protein / sample were determined using a multiplex assay containing a mixture of beads specific for each cytokine.
| Sample Source | Condition | IL-1β | IL-6 | TNFα |
|---|---|---|---|---|
| Serum# | Veh | 1.7 ± 0.7 | 0.61 ± 0.2 | 0.9 ± 0.2 |
| LPS | 49.6 ± 1.9* | 522.5 ± 24.1* | 11.6 ± 0.9* | |
| Spleen | Veh | 357.5 ± 8.8 | 3.5 ± 0.5 | 7.0 ± 0.4 |
| LPS | 1459.0 ± 19.3* | 518.1 ± 22.5* | 17.1 ± 0.8* | |
| Hypothalamus | Veh | 6.1 ± 0.5 | 1.0 ± 0.1 | 2.4 ± 0.6 |
| LPS | 30.1 ± 0.6* | 14.1 ± 1.1* | 5.9 ± 1.5* | |
| Hippocampus | Veh | 9.3 ± 0.7 | 1.5 ± 0.2 | 4.4 ± 1.2 |
| LPS | 54.2 ± 1.4* | 24.1 ± 1.5* | 8.6 ± 0.3* | |
| Brainstem | Veh | 17.4 ± 1.8 | 2.2 ± 0.3 | 4.6 ± 0.4 |
| LPS | 78.0 ± 9.0* | 19.1 ± 2.3* | 7.0 ± 0.4* |
Values are means ± Stdev (pg/mg) from n = 4 samples / condition. IL-1β: interleukin-1β; IL-6: interleukin-6; TNFα: tumor necrosis factor α
pg/ml; Veh: vehicle.
Asterisks (*) = p < 0.01.
Detection (“recovery”) of recombinant cytokines in whole brain homogenate ranged from 80 – 103 % of the amount spiked (Table 1, Table 2). When no brain homogenate was included in the sample, i.e., assay buffer only, no cytokines were detected. When assay buffer samples that had been spiked with recombinant cytokines were then assayed for a single analyte (IL-1β IL-6, or TNFα) using 1-plex kits, amounts detected ranged from 80 – 97 % of the amount spiked. Detection was 90 % or greater for those samples that had been spiked with 20 pg or more recombinant cytokine (Table 1). Similarly, 3-plex kits yielded detection percentages above 90 % for all but two spiked buffer samples (Table 2).
Each of the cytokines of interest was detected in whole brain homogenate samples that had not been spiked with recombinant cytokines (Table 1, Table 2). There were no statistically significant differences between the 1-plex and 3-plex assays in the amount of basal (endogenous) cytokines detected. Whole brain homogenate did not interfere with detection of recombinant cytokines. When the amount of total protein loaded into the sample was doubled, from 100 µg to 200 µg, the amounts of basal cytokine detected in unspiked samples ranged from 85 – 120 %, similar to the theoretical doubling of 100% detection (Table 1, Table 2).
Detection percentages for recombinant cytokines in spiked samples containing whole brain homogenates ranged from 80 – 103 %, irrespective of whether single analytes were assayed using 1-plex kits (Table 1) or multiple analytes were assayed using 3-plex kits (Table 2). Furthermore, when 200 µg of total protein was loaded into the sample, the percentages of the spiked amount detected were essentially the same as those determined from spiked samples that contained 100 µg total protein (Table 1, Table 2).
Under some conditions, calculations as used in this study based on “percent of total protein” ([(observed concentration)/(basal + added concentrations)] * 100) may give greater values for “cytokine recovery” than if values had been based on “percent of difference” calculations ([(observed concentration - basal)/(added concentrations)] * 100). The difference between these two methods of calculation is most apparent when concentrations of the spiked cytokine are lower than the basal concentration, i.e., when the spike consists of less cytokine protein than is already present in the sample. In this study, when whole brain homogenates were spiked with 5 pg of cytokine, detected cytokine values ranged from 89 to 103 % of the spike (across 1-plex and 3-plex kits). Using the same data, but calculating detection using a “percent of difference” approach, detected cytokine values ranged from 60 to 100 % of the spike (across 1-plex and 3-plex kits). Using the latter method, one concludes a lower “recovery” of known concentrations of cytokine. However, when the concentration of the spiked cytokine is greater than the basal amount of cytokine present in the sample, differences in results of detection between the methods of calculation converge and at higher concentrations disappear. For example, differences in “percent recovery” obtained by the two methods of calculation when the spike consisted of 20 or 50 pg of cytokine protein were about 5 percent, and values for “percent recovery” calculated by these two methods were identical when the cytokine spike was of 500 pg.
IP administration of LPS increased cytokine protein content in all samples assayed (Table 3). The most dramatic increases were in concentrations of IL-6, which, depending on the origin of the sample, ranged from about 9 (brainstem) to 870 (serum) fold. In every sample tested, IL-6 exhibited the highest magnitude of increase, whereas increases in TNFα were of the least magnitude. Increases in cytokines in the periphery (serum, spleen) were of greater magnitude than were increases for the same cytokines in discrete brain regions (Table 3).
DISCUSSION
During the last few years there have been several validation studies of the Luminex 100/200 multiplex bead array platform. A few of these studies have determined concentrations of multiple cytokines from the same sample [e.g., (Martins, Pasi et al., 2002;Pickering, Martins et al., 2002;De Jager, Velthuis et al., 2003;Khan, Smith et al., 2004;Bobrowski, McDuffie et al., 2005;McDuffie, Obert et al., 2006)]. Most validation studies have used serum/plasma or other biological samples obtained from peripheral organs of patients or laboratory animals. Several studies have used suspension bead array systems to analyze cerebrospinal fluid from patients with a variety of pathologies [e.g., (Buttram, Wisniewski et al., 2007)]. Although, one study has demonstrated the utility of this methodology for determining cytokine concentrations in the rat hippocampus (Hulse, Kunkler et al., 2004), little effort has been devoted to determination of the suitability of suspension bead arrays for quantification of cytokine protein concentrations in brain tissue. We believe this to be the first demonstration that cytokine protein can be reliably detected in small tissue homogenate samples obtained from discrete mouse brain regions using multiplex bead arrays.
In this present study, we did not compare results from multiplex bead assays with those obtained using ELISA. The primary research focus of our laboratory is on relative change in cytokine concentrations across time, rather than on direct comparisons of absolute values. However, recent literature suggests that multiplex bead array assays are generally comparable to ELISA with respect to sensitivity and reproducibility [e.g., (Pickering, Martins, Schroder, and Hill, 2002;De Jager, Velthuis, Prakken, Kuis, and Rijkers, 2003;Khan, Smith, Reda, Suffredini, and McCoy, Jr., 2004)], although kits from some manufacturers perform better than others (Liu, Xydakis et al., 2005). In some cases, very good correspondence between absolute values as determined by ELISA and multiplex bead arrays has been demonstrated (Khan, Smith, Reda, Suffredini, and McCoy, Jr., 2004). Collectively, these aforementioned studies demonstrate in a variety of diagnostic and research applications the utility of the multiplex suspension bead array assay.
Research interests in our laboratory focus on the role of cytokines in brain as mediators/regulators of complex physiological processes and behaviors. Using Bio-Plex kits, we detected IL-1β, IL-6, and TNFα in unspiked whole mouse brain homogenates. These results contribute to the literature demonstrating the presence of cytokine protein in healthy brain in the absence of pathology. Of particular interest to us is the increase in cytokine protein in discrete brain regions following peripheral administration of lipopolysaccharide (LPS). We demonstrate in this study that IP administration of 10 µg LPS increased IL-1β, IL-6, and TNFα in serum and spleen of C57BL/6J mice. These findings of LPS-induced increases in cytokine protein in mouse serum and spleen are in agreement with extant literature [e.g., (Amiot, Fitting et al., 1997;Ghezzi, Sacco et al., 2000;van Enckevort, Sweep et al., 2001;Juttler, Inta et al., 2007;Qin, Wu et al., 2007)]. However, we are unaware of assessment of cytokine protein in discrete mouse brain regions after LPS treatment. Our present results demonstrate that 4 h after LPS administration, concentrations of IL-1β, IL-6 and TNFα increase dramatically in mouse hypothalamus, hippocampus, and brain stem. These brain regions are involved in the regulation of multiple complex behaviors and physiological processes, including among others, activation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system activity.
We did not, in this study, determine the time course of LPS-induced increases in cytokine protein. Our samples were obtained at a single time point, 4-h after injection of vehicle or LPS. The cytokine cascade in response to LPS challenge is now considered textbook information. Very simply, LPS induces first TNF, which induces IL-1, which then induces IL-6 [see (Abbas, Lichtman et al., 1991)]. Although LPS is able to directly induce each of these aforementioned cytokines, and there are numerous feedback and feedforward regulatory loops, this generalized schema of the cytokine cascade is apparent in vivo and in vitro [e.g., (Chensue, Terebuh et al., 1991;Ulich, Guo et al., 1991;Amiot, Fitting, Tracey, Cavaillon, and Dautry, 1997;Jansky, Reymanova et al., 2003)]. Results obtained in our present study indicate that LPS-induced increases in IL-6 are of the greatest magnitude, whereas LPS-induced increases in TNF are of lowest magnitude, irrespective of the origin of the sample. These results are consistent with the known time course of the cytokine cascade after LPS challenge; protein concentrations of TNF and IL-1 are already subsiding when IL-6 peaks.
In conclusion, the Luminex platform is a cost effective tool for use in a variety of diagnostic and research applications. This platform provides a means by which multiple cytokines may be reliably detected from a single sample of small volume. We have demonstrated the assays are sensitive enough to detect basal cytokine concentrations in unstimulated whole mouse brain homogenates as well as in discrete mouse brain regions after LPS challenge. The assays are relatively simple to run and result in considerable time savings because a single multiplex assay may be used rather than multiple single assays. In addition, the ability to assay multiple analytes from the same sample represents a substantial reduction in the number of animals needed to complete a given study. In addition to reducing the ethical cost and financial expenditure associated with the purchase of animals, there are substantial cost savings in terms of the number of kits that need to be purchased. For example, on a per analyte basis, the cost of using 3-plex kits to determine concentrations of IL-1β, IL-6 and TNFα from the samples in this study was approximately 20% less than the cost of using 1-plex kits, and approximately 35% less than the cost of commercially-available ELISAs had we used them.
ACKNOWLEDGMENTS
We gratefully acknowledge the technical assistance of Ms. Jill Priestley and Ms. Ashley Talsma. Supported by: National Institutes of Health grants MH64843, HL080972, and the Department of Anesthesiology, University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- 1.Abbas AK, Lichtman AH, Pober J. Cellular and Molecular Immunology. Philadelphia: W. B. Saunders; 1991. Cytokines; pp. 225–243. [Google Scholar]
- 2.Amiot F, Fitting C, Tracey KJ, Cavaillon JM, Dautry F. Lipopolysaccharide-induced cytokine cascade and lethality in LT alpha/TNF alpha-deficient mice. Mol.Med. 1997;3:864–875. [PMC free article] [PubMed] [Google Scholar]
- 3.Bobrowski WF, McDuffie JE, Sobocinski G, Chupka J, Olle E, Bowman A, Albassam M. Comparative methods for multiplex analysis of cytokine protein expression in plasma of lipopolysaccharide-treated mice. Cytokine. 2005;32:194–198. doi: 10.1016/j.cyto.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Buttram SD, Wisniewski SR, Jackson EK, Adelson PD, Feldman K, Bayir H, Berger RP, Clark RS, Kochanek PM. Multiplex assessment of cytokine and chemokine levels in cerebrospinal fluid following severe pediatric traumatic brain injury: effects of moderate hypothermia. J Neurotrauma. 2007;24:1707–1717. doi: 10.1089/neu.2007.0349. [DOI] [PubMed] [Google Scholar]
- 5.Chensue SW, Terebuh PD, Remick DG, Scales WE, Kunkel SL. In vivo biologic and immunohistochemical analysis of interleukin-1 alpha, beta and tumor necrosis factor during experimental endotoxemia. Kinetics, Kupffer cell expression, and glucocorticoid effects. Am J Pathol. 1991;138:395–402. [PMC free article] [PubMed] [Google Scholar]
- 6.Dantzer R. Cytokine, sickness behavior, and depression. Neurol.Clin. 2006;24:441–460. doi: 10.1016/j.ncl.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Jager W, Velthuis H, Prakken BJ, Kuis W, Rijkers GT. Simultaneous detection of 15 human cytokines in a single sample of stimulated peripheral blood mononuclear cells. Clin.Diagn.Lab Immunol. 2003;10:133–139. doi: 10.1128/CDLI.10.1.133-139.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghezzi P, Sacco S, Agnello D, Marullo A, Caselli G, Bertini R. Lps induces IL-6 in the brain and in serum largely through TNF production. Cytokine. 2000;12:1205–1210. doi: 10.1006/cyto.2000.0697. [DOI] [PubMed] [Google Scholar]
- 9.Gibertini M. Cytokines and cognitive behavior. Neuroimmunomodulation. 1998;5:160–165. doi: 10.1159/000026332. [DOI] [PubMed] [Google Scholar]
- 10.Hulse RE, Kunkler PE, Fedynyshyn JP, Kraig RP. Optimization of multiplexed bead-based cytokine immunoassays for rat serum and brain tissue. J.Neurosci.Methods. 2004;136:87–98. doi: 10.1016/j.jneumeth.2003.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansky L, Reymanova P, Kopecky J. Dynamics of cytokine production in human peripheral blood mononuclear cells stimulated by LPS or infected by Borrelia. Physiol Res. 2003;52:593–598. [PubMed] [Google Scholar]
- 12.Juttler E, Inta I, Eigler V, Herrmann O, Maegele I, Maser-Gluth C, Schwaninger M. Neuronal NF-kappaB influences thermoregulation and survival in a sepsis model. J Neuroimmunol. 2007;189:41–49. doi: 10.1016/j.jneuroim.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 13.Khan SS, Smith MS, Reda D, Suffredini AF, McCoy JP., Jr Multiplex bead array assays for detection of soluble cytokines: comparisons of sensitivity and quantitative values among kits from multiple manufacturers. Cytometry B Clin.Cytom. 2004;61:35–39. doi: 10.1002/cyto.b.20021. [DOI] [PubMed] [Google Scholar]
- 14.Leon LR. Molecular Biology of Thermoregulation: Invited Review: Cytokine regulation of fever: studies using gene knockout mice. J.Appl.Physiol. 2002;92:2648–2655. doi: 10.1152/japplphysiol.01005.2001. [DOI] [PubMed] [Google Scholar]
- 15.Liu MY, Xydakis AM, Hoogeveen RC, Jones PH, Smith EO, Nelson KW, Ballantyne CM. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clin.Chem. 2005;51:1102–1109. doi: 10.1373/clinchem.2004.047084. [DOI] [PubMed] [Google Scholar]
- 16.Martins TB, Pasi BM, Pickering JW, Jaskowski TD, Litwin CM, Hill HR. Determination of cytokine responses using a multiplexed fluorescent microsphere immunoassay. Am J Clin.Pathol. 2002;118:346–353. doi: 10.1309/N0T6-C56B-GXB2-NVFB. [DOI] [PubMed] [Google Scholar]
- 17.McDuffie E, Obert L, Chupka J, Sigler R. Detection of cytokine protein expression in mouse lung homogenates using suspension bead array. J Inflamm.(Lond) 2006;3:15. doi: 10.1186/1476-9255-3-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nix B, Wild D. Calibration curve-fitting. In: Wild D, editor. The Immunoassay Handbook. New York: Nature Publishing Group; 2001. pp. 198–210. [Google Scholar]
- 19.O'connor KA, Holguin A, Hansen MK, Maier SF, Watkins LR. A method for measuring multiple cytokines from small samples. Brain Behav.Immun. 2004;18:274–280. doi: 10.1016/j.bbi.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Opp MR. Cytokines and sleep. Sleep Med.Rev. 2005;9:355–364. doi: 10.1016/j.smrv.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Osuchowski MF, Siddiqui J, Copeland S, Remick DG. Sequential ELISA to profile multiple cytokines from small volumes. J Immunol.Methods. 2005;302:172–181. doi: 10.1016/j.jim.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Pickering JW, Martins TB, Schroder MC, Hill HR. Comparison of a multiplex flow cytometric assay with enzyme-linked immunosorbent assay for auantitation of antibodies to tetanus, diphtheria, and Haemophilus influenzae Type b. Clin.Diagn.Lab Immunol. 2002;9:872–876. doi: 10.1128/CDLI.9.4.872-876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. GLIA. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toth LA, Opp MR. Infection and Sleep. In: Lee-Chiong T, Carskadon MA, Sateia M, editors. Sleep Medicine. Philadelphia: Hanley & Belfus, Inc.; 2002. pp. 77–84. [Google Scholar]
- 25.Turnbull AV, Rivier C. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: actions and mechanisms of action. Physiol.rev. 1999;79:1–71. doi: 10.1152/physrev.1999.79.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Ulich TR, Guo KZ, Remick D, del Castillo J, Yin SM. Endotoxin-induced cytokine gene expression in vivo. III. IL-6 mRNA and serum protein expression and the in vivo hematologic effects of IL-6. J Immunol. 1991;146:2316–2323. [PubMed] [Google Scholar]
- 27.van Enckevort FH, Sweep CG, Span PN, Demacker PN, Hermsen CC, Hermus AR. Reduced adrenal response to bacterial lipopolysaccharide in interleukin-6-deficient mice. J Endocrinol.Invest. 2001;24:786–795. doi: 10.1007/BF03343928. [DOI] [PubMed] [Google Scholar]