Abstract
Chance events such as seed dispersal determine the potential composition of plant communities, but the eventual assemblage is determined in large part by subsequent interactions among species. Postcolonization sorting also affects the ultimate composition of communities assembled by people for restoration, horticulture, or conservation. Thus, knowledge of the mechanisms controlling interspecific interactions in plant communities is important for explaining patterns observed in nature and predicting success or failure of utilitarian combinations. Relationships among species, especially those from studies of biological diversity and ecosystem functioning, are largely based on studies of short-lived, temperate-zone plants. Extrapolation to perennial plants in the humid tropics is risky because functional relationships among large-stature species change with time. Shifts in competitive relationships among 3 life forms—trees, palms, and perennial herbs—occurred during 13 yr in experimental tropical ecosystems. In 2 cases the novel competitive mechanism responsible for the shift was reduction in crown volume, and therefore light-capturing capability, of overtopping deciduous trees by intrusive growth from below a palm. In a third case, complementary resource use developed between 2 evergreen life forms (overstory tree and palm), probably because of differential nutrient acquisition. Species-level traits and adequate time for shifts in interspecific relationships to emerge are crucial for predicting community trajectories.
Keywords: complementarity, diversity, ecosystem functioning, fertile soil, plant competition
Design of sustainable ecosystems, whether for biodiversity conservation, economic gain, or restoration of ecosystem services, involves the assembly of plant communities comprising species of high ecological combining ability (1, 2). That combining ability is determined by interspecific interactions and refers to the ways and degree to which common resources, particularly light, water, and mineral nutrients, are shared. Does the nature of plant interactions remain relatively constant over time? If not, prediction of combining ability will require either case-specific long-term observation or thorough understanding of the mechanisms involved.
To examine the stability of plant interactions, and to reveal mechanisms driving any changes observed, we constrained the number of perennial life-form groups in simple experimental communities while varying the specific identity of 1 of them. The study site, a young alluvial terrace at La Selva Biological Station in the humid tropical lowlands of Costa Rica, was chosen because of its exceptionally fertile soil and warm, wet climate. By conducting experiments in an environment where plants experienced few abiotic constraints on growth we achieved results quickly and avoided the delays intrinsic to environments where plant growth is slow or where the ecological clock is reset annually.
The life forms selected are among those most common in mature forest at La Selva (3) and characteristic of forests in warm, wet climates on all continents. They are structurally and functionally very different from one another and included 3 native, fast-growing tree species capable of reaching the forest canopy: Hyeronima alchorneoides, Cedrela odorata, and Cordia alliodora; 1 alien palm, Euterpe oleracea, which has a native congener; and 1 native, giant perennial herb, Heliconia imbricata (all species are referred to hereafter by genus). The intent was to encompass some of the variability within the broad category of canopy-tree life form while holding the identity of the monocots (palm and herb) constant. Among other differences, Hyeronima (like the 2 monocots) is never leafless whereas the other 2 tree species are deciduous after attaining age 5–7 yr, Cedrela in the dry season and Cordia in the wet season. Tree seedlings were planted at high density (2,887 plants per hectare) in replicated 0.24-ha plantations (3 per tree species). Trees were lightly thinned periodically during the early years of the study to avoid stand stagnation while sustaining complete use of resources. Half of each plantation was retained as a single-species community (monoculture), and Euterpe and Heliconia were added to the other half, creating polycultures. Both monocots have very long leaves (Euterpe, ≈3 m; Heliconia, ≈2 m) and display their lamina almost vertically, giving them very high leaf area.
Results
To assess the performance of individual plant species as well as entire communities, we used aboveground net primary productivity (ANPP), which integrates net growth and biomass produced but lost during the growth-measurement interval (e.g., as mortality or litterfall). ANPP increased in all monocultures for the first 3–4 yr, then declined moderately (Hyeronima, from ≈30 to 18 Mg ha−1 yr−1; Cedrela, from ≈18 to 11 Mg ha−1 yr−1) or oscillated around relatively high levels (Cordia, 15; range 10–21 Mg ha−1 yr−1) for the next decade (Fig. 1). These values are at the middle to high end of the range typically reported for fast-growing trees in the tropics (4).
Fig. 1.
ANPP in monocultures and 3-species polycultures dominated by different tree species. (A) Hyeronima. (B) Cedrela. (C) Cordia. Open red symbols, tree in monoculture; filled red symbols, tree in polyculture; star, Heliconia; diamond, Euterpe; inverted triangle, polyculture total. Values are means ± SE from 3 blocks.
The time course of tree ANPP in polycultures was very different from that of monocultures in the case of the deciduous trees Cedrela and Cordia, whose ANPP in polyculture plummeted dramatically after 3 yr, even becoming negative (because of mortality) after 10 yr (Cedrela) or dropping to ≈1 Mg ha−1 yr−1 (Cordia). The start of the decline in tree ANPP in polyculture was synchronous with (in the case of Cedrela) or briefly preceded (in the case of Cordia) the peak of Heliconia ANPP, at which time Heliconia contributed 30–40% of ecosystem-level ANPP. By stand age 5 yr Heliconia, which has a basal meristem and whose maximum height is therefore biomechanically constrained, had been overtopped by the dense-canopied, evergreen Euterpe and no longer contributed to ANPP. Then, Euterpe ANPP soared as deciduous-tree ANPP plummeted, making Euterpe the dominant component of Cedrela and Cordia polycultures.
In the case of the evergreen Hyeronima, the time course of tree ANPP in polycultures was similar to that of monocultures, but the monocots responded very differently than when they were grown with the deciduous tree species. Heliconia never contributed measurably to total ANPP, and Euterpe growth was negligible for the first 6 yr beneath the Hyeronima canopy. Thereafter Euterpe began to grow more rapidly, eventually boosting ecosystem-level ANPP above levels in the Hyeronima monoculture—an example of complementary resource use.
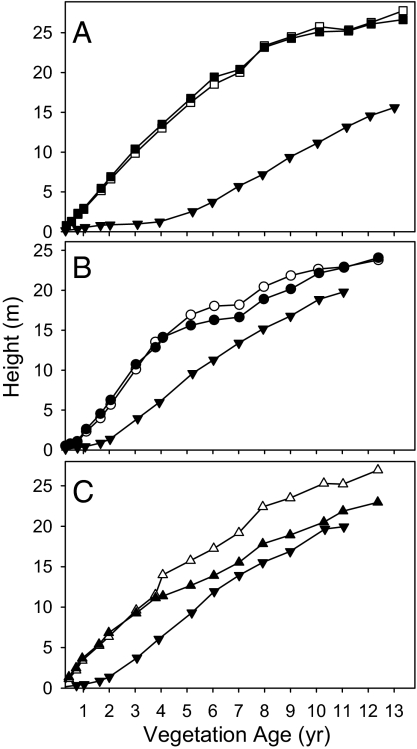
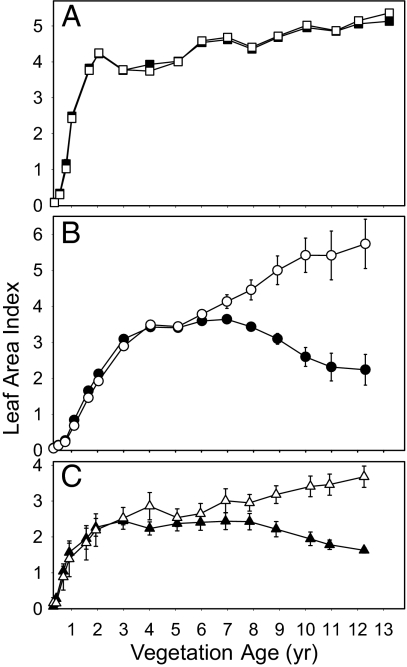
Even through the later years of the experiment, when growth of the deciduous trees in polycultures was stunted and many individuals died, the trees retained their canopy position as the tallest components in each of their respective polycultures (Fig. 2). Despite occupying the tallest stratum in these 3-life-form systems, the leaf area of the deciduous trees dropped well below their values in monocultures while the evergreen Hyeronima sustained high values in both monoculture and polyculture (Fig. 3). What resource or process limited Cedrela and Cordia productivity while the overtopped Euterpe thrived? There are 4 potential explanations: growth-limiting (i) light, (ii) nutrients, or (iii) water or (iv) differential herbivory or disease. Water and pests were assessed but not implicated [supporting information (SI) Text and Fig. S1].
Fig. 2.
Height relationships between tree species and palm (Euterpe, inverted triangle). (A) Hyeronima (squares). (B) Cedrela (circles). (C) Cordia (triangles up). Open symbols are trees in monoculture; filled symbols are trees in polycultures. Values are means of all individuals greater than or equal to the third quartile in each of 3 blocks. SEs are too small to show at this scale.
Fig. 3.
Leaf area index of tree species grown in monoculture (open symbols) and polyculture (filled symbols). (A) Hyeronima. (B) Cedrela. (C) Cordia. Leaf area index is leaf surface area, 1-sided, per unit area of ground. Values are means ± SE from 3 blocks.
Given the marked growth decline of the tallest components in the Cedrela and Cordia polycultures, it was logical to seek an explanation in competition for nutrients. After the expected rise in soil nutrients from the burning of cut vegetation that accompanied site preparation, most measures of soil fertility declined over time under all species (Fig. S2). Nevertheless, concentrations of extractable soil nutrients did not drop to critically low levels (5, 6). Furthermore, despite the major physiognomic and floristic differences between monocultures and polycultures, differences among species in concentrations of foliar nitrogen (N) and phosphorus (P) were consistent throughout the 13-yr study, regardless of the community in which they were located: tree species alone or in polyculture, and Euterpe and Heliconia grown with any of the 3 tree species (Fig. S3). Foliar N and P concentrations of all species were high in comparison with data from many tropical forests (7). Thus, there is no compelling evidence indicating that competition for N or P led to Euterpe dominance in the Cedrela and Cordia polycultures.
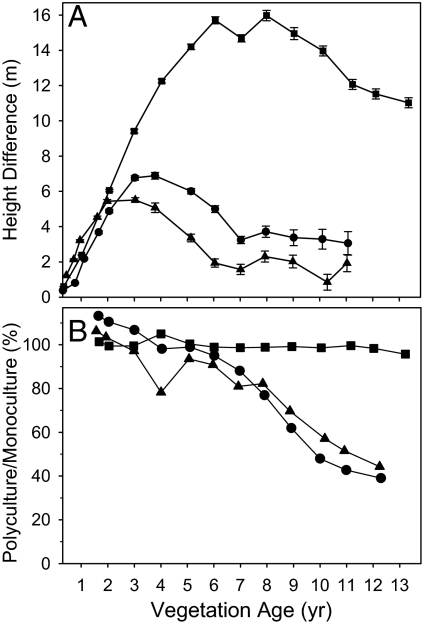
Reduced light capture proved to be the primary mechanism causing ANPP of the overtopping Cedrela and Cordia trees to decline: Euterpe reduced the leaf area of both of these tree species by invading their crowns from below. The result was a reduction in crown volume, and therefore leaf area, of both Cedrela and Cordia (Fig. 4). These 2 species are deciduous for part of each year, which provides the understory Euterpe (an evergreen, like all palms) with full access to sunlight during the time of tree leaflessness and facilitates its growth.
Fig. 4.
Bottom-up tree crown compression by understory palm. (A) Difference between height of tree in polyculture and coplanted palm. (B) Ratio of mean leaf area index of trees in monoculture to their leaf area index in polyculture. Squares, Hyeronima; circles, Cedrela; triangles, Cordia.
Although light was the resource that limited tree growth in polycultures containing Cedrela and Cordia, the evergreen Hyeronima did not suffer commensurate losses of leaf area in polycultures (Fig. 3). Nevertheless, the nutritional status of Hyeronima did change and may foreshadow its eventual fate. Foliar concentrations of N (but not P) in Hyeronima leaves declined significantly (P < 0.001 for all regressions) and at the same rate in monoculture and polyculture (Fig. 5). That decline might be explained partially by a dilution effect, because LAI (leaf surface area, 1-sided, per unit area of ground) increased in both monoculture and polyculture by a little more than 11%, while N concentrations declined by a little more than 20%. The remaining decline in foliar N (≈45% of the total) is almost certainly a consequence of lower rates of N mineralization under Hyeronima, whether in monoculture or polyculture, than under the other 2 tree species (8). The more-rapid decline in foliar N than of P led to a decline of N:P from ≈40 (molar basis) to values in the range of ≈20–27 (Fig. 5C); a ratio <31 is indicative of N limitation to growth (provided that N or P is limiting) (9, 10). Thus, competition between Hyeronima and Euterpe for ever-smaller supplies of N could lead to slower growth of Hyeronima and eventually to the same crown invasion from below that led to the demise of Cedrela and Cordia in their respective polycultures.
Fig. 5.
Foliar nutrients in Hyeronima. Shown are nitrogen (A) and phosphorus (B) (both on mass basis), where filled circles are analyses of bulk leaf samples (n = 3) from monocultures and other symbols are disks punched from lamina free of large veins. Values are means ± SE. Squares are samples from monoculture; diamonds are from polyculture. Nitrogen regressions for disks are not different between monoculture and polycultures; bulk samples differ in intercept from the disk samples, as expected (P = 0.007), but not slope (bmono = −0.091 disks and −0.106 bulk samples; bpoly = −0.097; difference test P = 0.776). N values from disks for age 8 yr were not included in calculation of regressions because of uncertainty of their validity. (C) Ratio of nitrogen to phosphorus, molar basis, where dotted line indicates level below which N may be limiting (9, 10).
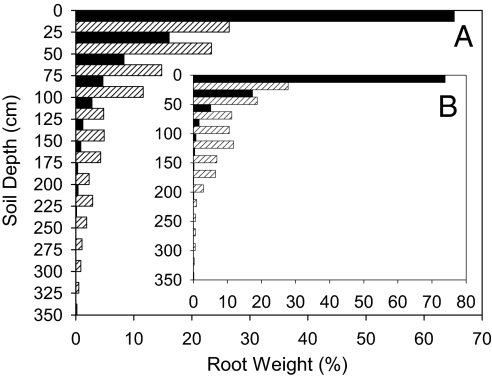
A second possible outcome for the Hyeronima polyculture is coexistence of Hyeronima and Euterpe in a 2-tiered community. Hyeronima trees attain heights of 50 m (3) whereas Euterpe is biomechanically constrained to ≈25 m. Hyeronima may maintain its considerable height advantage over Euterpe, precluding crown invasion from below by the palm. Coexistence might also be fostered by spatial partitioning of soil by root systems. The burst of growth by Euterpe in Hyeronima polycultures that only began ≈6 yr into the study (Fig. 1A) led us to investigate that possibility through root excavations. Hyeronima roots dominated the surficial horizons, whereas Euterpe roots dominated lower horizons and extended deeper into the soil (Fig. 6). Furthermore, Hyeronima (after age ≈7 yr) appeared to be N limited (Fig. 5C) whereas Euterpe appeared to be P limited (Fig. S3). Continued height growth by Hyeronima, spatial partitioning of soil, and different N-P stoichiometries could sustain a dual-life-form ecosystem.
Fig. 6.
Vertical distribution of tree (Hyeronima; filled bar) and palm (Euterpe; hatched bar) roots in 12-yr-old polyculture. Each pit had a surface area of 1.0 × 2.0 m. Pits were excavated in 2 blocks (A and B). Total root biomass was 7,357 g (Hyeronima) and 870 g (Euterpe) in pit A and 6,954 g (Hyeronima) and 1,077 g (Euterpe) in pit B.
Discussion
The important role of species traits became readily apparent in our experiments. The evergreen Hyeronima shaded the understory palm throughout the year, whereas the other 2 tree species, both deciduous, underwent several weeks of leaflessness annually, exposing the evergreen palm to full sunlight. Thus, the major trait that influenced our findings was phenology: an evergreen dominant gave a result that differed markedly from that yielded by 2 deciduous tree species. Despite their common trait of deciduousness, Cedrela and Cordia differ strongly in many other traits. For example, their leaf losses are not synchronous—one (Cedrela) is dry-season deciduous, and the other (Cordia) loses its leaves in the rainy season. Furthermore, they differ in leaf-level N use efficiency (11), their root systems and crowns develop very differently (12), and they are vulnerable to different herbivores against which they defend themselves using dissimilar mechanisms. The implication is that there is not a unique outcome for each and every combination of species, but that a particular key trait (leaf phenology in this case) can dominate the trajectory of ecosystem development and the eventual outcome of interspecific competition. Identification of those key traits, rather than endless trial and error, is the challenge.
To what extent might this newly revealed mechanism of plant competition—reduction of the light-capturing capacity of a tree crown by invasion from below—be a general phenomenon? Among life forms, palms are exceptionally well-suited to penetrate tree crowns. The single apical bud of a palm is extremely well-protected (13), and although the fibrous, sturdy fronds may fray they do not manifest the shyness that characterizes the bud-tipped edges of abrading dicotyledonous tree crowns (14). Furthermore, their evergreen habit and relatively low requirement for P (at least among the handful of species studied, e.g., refs. 15 and 16 and Fig. S3) make palms well-suited for opportunistic growth in the understory. Nevertheless, although invasion from below may be a behavior for which many palms are exceptionally well-adapted, there is no reason to think that it is a unique phenomenon. For example, an evergreen dicotyledonous tree having sturdy branches and well-protected buds might well exhibit the same competitive mechanism.
In wet forests like La Selva, where deciduous tree species are relatively uncommon (perhaps 1 individual per hectare), the temporal gaps created by periods of overstory leaflessness may not provide major opportunities for competition from below, but in drier environments it may be a major process structuring the canopy. In 3 Panamanian forests receiving 71%, 64%, and 52% of La Selva's mean annual rainfall, the fraction of the canopy that is deciduous is 4%, 10%, or 19%, respectively (17), affording substantial opportunity for growth from below. In an extreme case of deciduousness, arborescent cacti reach the canopy of dry forest in Mexico by sustaining growth during periods when the overtopping canopy is leafless (18).
Interactions among species having access to a common pool of resources can change dramatically with time (19, 20), a phenomenon that has important implications for restoration and management. Even under the unconstrained conditions for plant growth at our study site, it was several years into the 13-yr study before important shifts in interspecific relationships emerged. Complementary resource use by co-occurring species does promote enhanced ecosystem productivity, but resource sharing and partitioning are not inevitably sustained as plants grow. Furthermore, a complementary relationship can become competitive through unanticipated mechanisms: 2 of our tree species were starved for light not by being overtopped but by having their canopy space reduced 2- to 5-fold by invasion from below. As demonstrated by our findings, traits of individual species, or of narrowly defined functional groups, can lead to shifts in ecosystem functioning over decadal time scales. Those attempting to build ecosystems for conservation, restoration, or production must consider not only static measures of potential combining ability, such as phenology, growth rates, stature, architecture, and resource requirements, but also dynamic components including changes in relationships among species as they grow at different rates and use resources in different ways.
Materials and Methods
The research was conducted at La Selva Biological Station of the Organization for Tropical Studies, Inc.: 10° 26′ N; 83° 59′ W; 41 m above sea level; mean annual rainfall and temperature 3.9 m and 25°C, respectively (www.ots.ac.cr). The 8-ha site was on a recent alluvial terrace derived primarily from volcanic material (21). The soil (eutric Hapludand) (22) was deep (≫4 m) and very well-drained and had high base cation saturation and high capacity to stabilize organic matter and retain P and water (30–34% by volume of residual water content).
The study involved 3 life forms, or functional groups: dicotyledonous canopy trees (3 species) and 2 perennial monocotyledons, a palm and a large-stature herb. The tree species differed in phenology, physiology, and architecture. H. alchorneoides Allemão (Euphorbiaceae) is an evergreen species whose leaf size declines >4-fold from youth (when leaves average ≈280 cm2) to adulthood (23); it forms a dense canopy that captures most incident radiation. C. odorata L. (Meliaceae) has long (0.5–1.0 m) compound leaves containing 20–40 leaflets, each ≈40 cm2. Cedrela is rarely leafless before age ≈5 yr, after which it becomes dry-season deciduous. C. alliodora (R. & P.) Cham. (Boraginaceae) has simple leaves (≈30 cm2) and a tiered, open crown that permits substantial light to penetrate to the understory; it is evergreen in youth but wet-season deciduous after age 5–7 yr. The ranges of both deciduous tree species, Cedrela and Cordia, extend well into much drier climates, indicating that their leaf loss in the wet climate of La Selva may be a trait carried over from selection in dry forests. The 3 tree species differ in leaf-level nutrient use efficiency (NUE) of N (Cedrela > Hyeronima = Cordia) but not P (11), and in whole-tree NUE of P (Hyeronima > Cedrela = Cordia) but not N (24). Subcanopy palms are an important life form at La Selva, where they constitute 3 of the 4 most common species on rolling terrain (3). The palm we used (E. oleracea Mart; Arecaceae) was the only alien species in the experiment; it is native to floodplains of lower Amazonia, but it has a native congener (Euterpe precatoria) in the local forest. One large Euterpe typically has ≈20 ramets, each of which supports numerous pinnate leaves ≈3 m long; the 40–80 leaf segments, each ≈1 m long, droop vertically, and their combined number, size, and orientation result in extremely high leaf area (LAI of Euterpe reaches 10). The other monocot [H. imbricata (Kuntze) Baker; Heliconiaceae] is a giant perennial herb that produces multiple monocarpic shoots. Its leaf blades are ≈0.3 m wide and 2 m long. Like Euterpe, the vertical orientation of its foliage results in extremely high leaf area (LAI of up to 8). Because of its basal meristem and the mass of its leaf blades, the height of Heliconia is biomechanically constrained to ≈6 m. All 5 species used in the experiments occur naturally and grow rapidly on fertile, well-drained alluvium.
Before the start of the experiment existing vegetation (an abandoned cacao plantation) was felled, commercial logs were removed, and slash was burned. The experimental design was a split plot of 3 blocks. Data were analyzed (SAS version 9.1, Mixed Procedure) with blocks as a random factor, tree species as the whole-plot factor, and treatment as the subplot factor. Because of the multiple measurements over time at each block–tree species–treatment combination, repeated-measures analyses were performed allowing for serial correlation in the covariance structure. Each block contained 6 plots, each 30 × 40 m, plus exterior rows of the same species to act as a buffer between treatments and surrounding vegetation, which was maintained at the same stature as the experimental vegetation. Each plot was surrounded by a root-barrier cloth, buried vertically in the soil to a depth of 1 m. Two contiguous plots (60 × 40 m) in each block were planted to the same tree species with individuals 2.0 m apart. One plot of each pair was left as a monoculture. The other plot was enriched to become a polyculture, created by additive planting (25) of the 2 monocots, the palm (Euterpe) at one-fourth the tree density and, a year later, the large, perennial herb (Heliconia) at one-half the tree density. Natural colonists were removed as they appeared, a process that was unnecessary after about age 2 yr in any plantation containing Hyeronima or in any polyculture.
Heights and diameters of all plants in the core 30 × 30 m of each plot were measured annually (each trimester in the first 2 yr). Heights were measured to the crown apex (trees) or top of the tallest unfurled leaf bud (monocots). Diameters were measured at the base of trees, just above the root collar swelling, until they attained a height >1.5 m, after which diameter was measured at the standard height of 1.3 m. Counts of all shoots from the same ramet and their sizes were recorded for the monocots. The final growth measurements of Euterpe in Cedrela and Cordia polycultures were made at age 11 yr, when the trajectory of tree growth in those stands became obvious and irreversible (near-zero ANPP).
At least annually 6–18 individuals of each species were harvested for biomass assessments. Harvesting was done from 5-m border zones designated for that purpose along the edges of each plot and outside of data-gathering zones. Plants were separated into leaves, branches, bole, petiole or petiolule, rachis (Cedrela and Euterpe), and reproductive structures. Parts <1 kg were taken in their entirety as a sample; components of larger mass were pooled, mixed, and subsampled (≈1 kg) for analysis. Specific leaf area (SLA, cm2/g) was determined by measuring the area (to 0.01 cm2; LiCor 3100 area meter) of fresh leaf samples (≥30 leaves or leaflets per subsample) and determining their mass after oven-drying for ≥24 h at 70°C. Plant dimensions were converted to biomass by using allometric equations involving 258–379 individuals of each tree species and Euterpe (26) and unpublished equations in the case of Heliconia. The mass of each plant was estimated, and then these values were summed over the entire plot and divided by plot area to obtain biomass/area. Total leaf area was estimated by converting stand-level leaf biomass (by species, from allometry) to leaf area based on measurements of SLA.
Litterfall was collected biweekly from 4 screen-bottomed, 50 × 173-cm traps in each plot. Traps were elevated 10 cm above the soil to reduce losses to decay between collections. Litter was separated by species and component (leaves, rachises, twigs, reproductive parts). Early in the study the trees were lightly thinned at 2- to 3-yr intervals to ensure full use of resources while avoiding stand stagnation. Trees (primarily Cedrela) whose main stems divided below the height of diameter measurement were pruned when young to a height of 1.5 m. ANPP was calculated as the difference between successive stand-level biomass determinations plus litterfall, increment of trees that died in the interval between measurements (whether from natural causes or thinning), and pruned tissues.
Soil was sampled annually at depths of 0–10, 10–25, 25–70, and 70–120 cm (70–155 cm in the 6 plots of 1 of the 3 blocks). At each depth, 1 composite sample (n = 3 cores) was taken from each of the 3 plots per treatment. Samples were air-dried and ground to pass a 2-mm sieve. Soil pH was measured in water and KCl by using a soil-to-solution ratio of 1:2.5. Organic carbon (C) was determined by wet digestion (Walkley-Black). Calcium (Ca), magnesium (Mg), and exchangeable acidity [aluminum (Al) + hydrogen (H)] were extracted with unbuffered 1 M KCl and determined by atomic absorption spectrometry (Ca and Mg) or titration (Al+H) (27). Potassium (K) and P were extracted by using an Olsen solution (sodium bicarbonate at pH 8.5) modified by addition of EDTA and superfloc (28) and determined by atomic absorption spectrometry and the ascorbic acid-molybdate method, respectively. Effective cation exchange capacity (effective CEC) was calculated as the sum of extractable cations plus exchangeable acidity. CEC was also determined by saturation with NH4OAc at pH 7 (29). Total N was measured 4 times over the study period by using elemental analyzers.
Foliar nutrients were determined annually in subsamples of material taken for biomass assessments and in 8 of 13 yr in disks (each 0.25 cm2; samples of n ≥ 600) punched from intervein lamina. Samples were oven-dried at 70°C for ≥24 h, finely ground in a Wiley mill, and digested following a Kjeldahl protocol; N and P in the digests were analyzed by automated colorimetry.
We excavated roots from 2 large (1.0 × 2.0 m) soil pits in 12-yr-old Hyeronima polycultures. Each pit was positioned midway between 2 rows of trees such that it intersected root systems of vigorous Hyeronima and Euterpe. The pits were excavated by 0.25-m layers to the deepest extent of roots (3.5 or 3.75 m). Soil from each layer was sieved (0.1-cm mesh screens) to extract roots, which were then sorted into 3 diameter classes (0–0.2, >0.2–0.5, and >0.5 cm), separated by species, oven-dried at 70°C, and weighed.
Supplementary Material
Acknowledgments.
We thank R. Bedoya, S. W. Bigelow, M. Cifuentes, J. P. Haggar, and A. Hiremath for field and laboratory work and supervision; the Organization for Tropical Studies, Inc., for logistical support; T. G. Cole, B. Ogut, J. Baldwin, and P. Satti for data management and analyses; and E. Bruna, K. Kitajima, F. E. Putz, E. A. G. Schuur, and 2 anonymous reviewers for comments on the manuscript. This work was supported by the National Science Foundation, the Andrew W. Mellon Foundation, the United States Department of Agriculture Forest Service, and Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807216105/DCSupplemental.
References
- 1.Harper JL. Population Biology of Plants. London: Academic; 1977. [Google Scholar]
- 2.Bradshaw AD. In: Restoration Ecology: A Synthetic Approach to Ecological Research. Jordan WRI, Gilpin ME, Aber JD, editors. Cambridge, UK: Cambridge Univ Press; 1987. pp. 23–30. [Google Scholar]
- 3.McDade LA, Bawa KS, Hespenheide HA, Hartshorn GS, editors. La Selva: Ecology and Natural History of a Neotropical Rain Forest. Chicago: Univ of Chicago Press; 2004. pp. 73–89.pp. 350–378. [Google Scholar]
- 4.Varmola M, Del Lungo A. Planted Forests Database: Structure and Contents. Rome: Food and Agriculture Organization; 2003. Planted Forests and Trees Working Papers 25. [Google Scholar]
- 5.Poudel DD, West LT. Soil development and fertility characteristics of a volcanic slope in Mindanao, the Philippines. Soil Sci Soc Am J. 1999;63:1258–1273. [Google Scholar]
- 6.Parfitt RL, et al. N and P in New Zealand soil chronosequences and relationships with foliar N and P. Biogeochemistry. 2005;75:305–328. [Google Scholar]
- 7.Townsend AR, Cleveland CC, Asner GP, Bustamante MMC. Controls over foliar N:P ratios in tropical rain forests. Ecology. 2007;88:107–118. doi: 10.1890/0012-9658(2007)88[107:cofnri]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Ewel JJ. Species and rotation frequency influence soil nitrogen in simplified tropical plant communities. Ecol Appl. 2006;16:490–502. doi: 10.1890/1051-0761(2006)016[0490:sarfis]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 9.Koerselman W, Meuleman AFM. The vegetation N:P ratio: A new tool to detect the nature of nutrient limitation. J Appl Ecol. 1996;33:1441–1450. [Google Scholar]
- 10.Aerts R, Chapin FS., III The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. Adv Ecol Res. 2000;30:1–67. [Google Scholar]
- 11.Hiremath AJ. Photosynthetic nutrient-use efficiency in three fast-growing tropical trees with differing leaf longevities. Tree Physiol. 2000;20:937–944. doi: 10.1093/treephys/20.14.937. [DOI] [PubMed] [Google Scholar]
- 12.Haggar JP, Ewel JJ. Primary productivity and resource partitioning in model tropical ecosystems. Ecology. 1997;78:1211–1221. [Google Scholar]
- 13.Corner EJH. The Natural History of Palms. Berkeley: Univ of California Press; 1966. [Google Scholar]
- 14.Putz FE, Parker GG, Archibald RM. Mechanical abrasion and intercrown spacing. Am Mid Nat. 1984;112:24–28. [Google Scholar]
- 15.Sanchez PA, Salinas JG. Low-input technology for managing oxisols and ultisols in tropical America. Adv Agron. 1981;34:279–406. [Google Scholar]
- 16.Schroth G, Elias MEA, Macêdo JLV, Mota MSS, Lieberei R. Mineral nutrition of peach palm (Bactris gasipaes) in Amazonian agroforestry and recommendations for foliar analysis. Eur J Agron. 2002;17:81–92. [Google Scholar]
- 17.Condit R, et al. Quantifying the deciduousness of tropical forest canopies under varying climates. J Veg Sci. 2000;11:649–658. [Google Scholar]
- 18.Lerdau MT, Holbrook NM, Mooney HA, Rich PM, Whitbeck JL. Seasonal patterns of acid fluctuations and resource storage in the arborescent cactus Opuntia excelsa in relation to light availability and size. Oecologia. 1992;92:166–171. doi: 10.1007/BF00317359. [DOI] [PubMed] [Google Scholar]
- 19.Rejmánek M, Lepš J. Negative associations can reveal interspecific competition and reversal of competitive hierarchies during succession. Oikos. 1996;76:161–168. [Google Scholar]
- 20.Hooper DU, Dukes JS. Overyielding among plant functional groups in a long-term experiment. Ecol Lett. 2004;7:95–105. [Google Scholar]
- 21.Sollins P, Sancho F, Mata R, Sanford RL., Jr . In: La Selva: Ecology and Natural History of a Neotropical Rain Forest. McDade LA, Bawa KS, Hespenheide HA, Hartshorn GS, editors. Chicago: Univ of Chicago Press; 2004. pp. 34–53. [Google Scholar]
- 22.Weitz AM, Grauel WT, Keller M, Veldkamp E. Calibration of time domain reflectometry technique using undisturbed soil samples from humid tropical soils of volcanic origin. Water Resour Res. 1997;33:1241–1249. [Google Scholar]
- 23.Reich A, Holbrook NM, Ewel JJ. Developmental and physiological correlates of leaf size in Hyeronima alchorneoides (Euphorbiaceae) Am J Bot. 2004;91:582–589. doi: 10.3732/ajb.91.4.582. [DOI] [PubMed] [Google Scholar]
- 24.Hiremath AJ, Ewel JJ, Cole TG. Nutrient use efficiency in three fast-growing tropical trees. For Sci. 2002;48:662–672. [Google Scholar]
- 25.Snaydon RW. Replacement or additive designs for competition studies? J Appl Ecol. 1991;28:930–946. [Google Scholar]
- 26.Cole TG, Ewel JJ. Allometric equations for four valuable tropical tree species. For Ecol Manage. 2006;229:351–360. [Google Scholar]
- 27.Thomas GW. In: Methods of Soils Analysis. Part 2. Page AL, Miller RH, Keeney DR, editors. Madison, WI: Am Soc of Agronomy, Soil Sci Soc of Am; 1982. pp. 159–165. [Google Scholar]
- 28.Hunter AH. International Soil Fertility Evaluation and Improvement Laboratory Procedures. Raleigh: North Carolina State Univ; 1974. [Google Scholar]
- 29.Soil Survey Staff. Soil Survey Laboratory Methods Manual. Washington, DC: United States Department of Agriculture, Natural Resources Conservation Service; 1996. Soil Survey Invest Rep 42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.