Abstract
The condensation, evaporation, and repartitioning of semivolatile organic compounds (SVOCs) in the atmosphere depends both on the phase of condensed material and the effective condensed phase vapor pressures of the SVOCs. Although direct measurements of vapor pressures of individual SVOCs exist, there are limited measurements of how the properties of a given compound changes in mixtures of multiple components that exist in the atmosphere. Here, the evaporation behavior of mixtures of dicarboxylic acids, which are common atmospheric aerosol constituents, is investigated. These measurements demonstrate that complex mixtures of the individually solid organic compounds take on liquid-like properties. Additionally, the vapor pressures of individual components show strong, identity-dependent deviations from ideality (i.e., Raoult's Law), with the vapor pressures of the smaller, more volatile compounds decreased significantly in the mixtures. The addition of an inorganic compound (NaNO3) further influences the nonideal behavior, again in a compound-specific manner. These results suggest that nonideal behavior of particle-phase compounds influences the abundances of organic aerosol observed in the atmosphere and in the laboratory.
Keywords: vapor pressures, activity coefficients, particulate matter, partitioning theory
Aerosols in the atmosphere are of great importance owing to their influence on global climate, precipitation, visibility, and human health (1). To assess the specific impacts of aerosols, it is necessary to have knowledge of the spatial and temporal variability of their amounts, size, chemical composition, and phase. The majority of submicrometer atmospheric aerosols are of mixed organic/inorganic composition (2, 3), and many of the thousands of organic compounds that comprise aerosols are derived from condensation of secondary volatile organic compounds (SVOCs) that are produced from gas-phase oxidation of volatile precursor gases (4). The formation and transformation of organic aerosol (OA) depends explicitly on the properties of the individual aerosol components (e.g., vapor pressure) and of the aerosol as a whole (e.g., phase). It is often assumed that the organic aerosols are liquids and that the individual components are not significantly affected by the presence of the other components, i.e., behave ideally. However, scant experimental evidence exists that clearly demonstrates the phase of atmospheric aerosols, and there is relatively little information on how the physical properties of individual SVOCs are modified within the chemically complex aerosols, i.e., the potentially nonideal behavior.
Several recent studies indicate that absorptive partitioning models of secondary organic aerosol formation that are based on parameterized laboratory aerosol yields underpredict the amount of OA observed in the atmosphere by factors of ≈10–100; this is the case for both downwind of urban sites and in the free troposphere (5–7). Models of OA formation based on detailed gas-phase chemical mechanisms using estimated gas-particle partitioning coefficients, assumed to behave ideally, also significantly underestimate observed OA abundances (8). The reasons for this discrepancy remain unclear, but may in part be related to improper treatment of organic aerosol (9). These differences hamper our accurate modeling of atmospheric abundances and properties of organic aerosols. For example, the ability of global models to critically assess the effect of aerosols on clouds are diminished because the organic fraction of particles in models may be significantly underestimated and the ability of aerosols to nucleate cloud droplets depends importantly on their composition (10). It is therefore crucial that the partitioning of SVOCs to atmospheric aerosol be better quantified.
Here, we consider the potentially important role of nonideal behavior of individual compounds within organic mixtures in an experimental framework. Our study is not meant to be comprehensive of all of the possible atmospheric aerosols and their components, but is meant to show the issues that need to be considered for an accurate calculation of atmospheric organic aerosols. We report on measurements of the temperature-dependent evaporation behavior of multicomponent mixtures of dicarboxylic acids that are commonly found in atmospheric organic aerosols. These measurements show that sufficiently complex mixtures of the individually solid organic compounds evaporate in a manner consistent with their phase being liquid-like. This behavior persists even when a nonvolatile inorganic salt is added to the mixture. Furthermore, comparison between the measured temperature-dependent evaporation rates (or equivalent vapor pressures) of the organic compounds in the mixtures with those of the pure compounds suggests that the common assumption that Raoult's Law can be used to describe the gas-particle partitioning of individual compounds in atmospheric aerosols may not be valid. The activity coefficients derived from these measurements indicate that commonly used activity coefficient estimation methods may only have limited accuracy in their ability to predict individual compound properties in complex organic mixtures. Implications of these results toward our understanding of gas-particle partitioning of organic compounds will be discussed.
We investigated the evaporation behavior of multicomponent mixtures by using a temperature-programmed desorption (TPD) method (see Methods Summary; refs. 11 and 12). Although we have previously applied the TPD method to the measurement of individual compound vapor pressures, here, we considered the behavior of a 9-component mixture of the C3-C10 and C12 straight-chain dicarboxylic acids, which are common constituents of atmospheric aerosols (13). Additionally, organic aerosol is relatively nonhygroscopic (14) and thus the behavior of these dry diacid mixtures can be considered as reasonably representative of atmospheric organic aerosols.
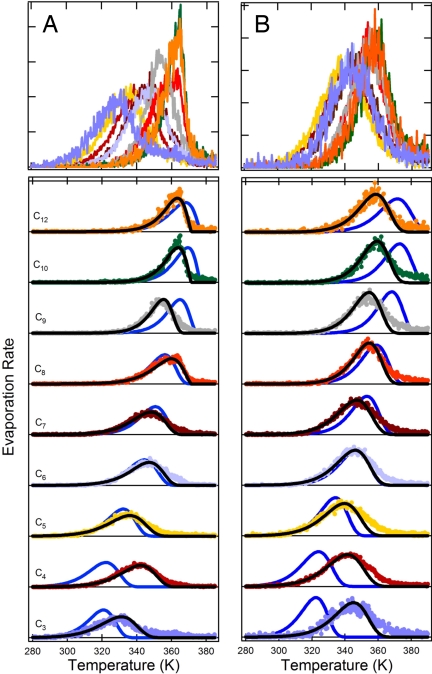
For this mixture, each of the individual components exhibited a unique desorption profile; the individual diacids were effectively distilled from the mixture (Fig. 1A). The distillation was observed even though the individual diacids are all solids. This behavior shows that the sample was continuously well-mixed and, given the timescales of our experiments (<1 h), this behavior would not have been observed if the 9-component mixture were actually a solid, as is the case for binary and ternary mixtures of the same diacids (C.D.C., E.R.L., and A.R.R., unpublished data). The observations are consistent with a mixing timescale of ≈10 s, estimated from τ ≈ r2/Di, where Di is the diffusion coefficient in the liquid-like mixture (Di ≈ 10−9 m2/s for liquids) (15), similar to the time between temperature steps. If our mixture were a solid, it would have required ≈104-105 longer times to show the observed behavior. In addition, the multicomponent mixtures did not deposit in our system as well-formed cones, in contrast to the pure diacids or binary or ternary mixtures (12). Instead, the samples appeared more spherical, thus providing a visual indication of the liquid-like nature of the mixtures. TPD measurements by Ziemann and coworkers on laboratory-generated secondary organic aerosol formed in the reaction of a few alkenes with NO3 and of alkanes with OH also show evidence for liquid-like behavior (i.e., distillation) (16, 17), although the shapes of their desorption curves did not lend themselves to a straightforward interpretation, possibly as a result of contributions of multiple compounds to the observed signal at a given m/z.
Fig. 1.
The measured desorption profiles for each of the 9 diacarboxylic acids in the multicomponent mixtures. (A) The measured desorption profiles for the purely organic mixture are shown (solid points) along with the model results assuming ideal behavior (solid blue lines) and for the fit to the measurements (solid black lines). The y axis corresponds to the measured evaporation rate. Top shows the desorption profiles for the individual compounds in a single graph to emphasize the distillation behavior. (B) Same as A, but for the equimolar organic/inorganic mixture. We suspect that the slight disagreements between model and measurement that remain at higher temperatures are the result of the evaporated molecules sticking in the ion drift tube.
Our measurements therefore provide direct evidence that the nonaqueous organic fraction of atmospheric aerosols, which certainly contain many more than 9 components, likely behave as well-mixed, liquid-like phases. Thermodynamic theory predicts that mixtures composed of hundreds of individually solid compounds should exhibit liquid-like behavior due to mutual melting point depression (18), but this experimental demonstration is nonetheless important given that liquid-like behavior is a general assumption in gas-particle-partitioning theory (19).
The observed desorption profiles have been quantitatively interpreted by using a multicomponent evaporation model (see Methods Summary). It is found that the shapes of the individual calculated desorption profiles compared well with the observations when ideal behavior was assumed for the mixture (Fig. 1A). The ideal model used “subcooled liquid” vapor pressures (pL0), derived from our previous measurements of the solid pure compound vapor pressures (pL0), where
and ΔSfus is the entropy of fusion and Tm is the melting temperature (12, 20–22) [see supporting information (SI) Text for ΔSfus and Tm values and Table S1]. However, although the shapes of the desorption profiles compared favorably, to obtain quantitative agreement with the measurements, we found that it was necessary to adjust the temperature-dependent liquid vapor pressures in the model from their pure values (see SI Text and Table S2).
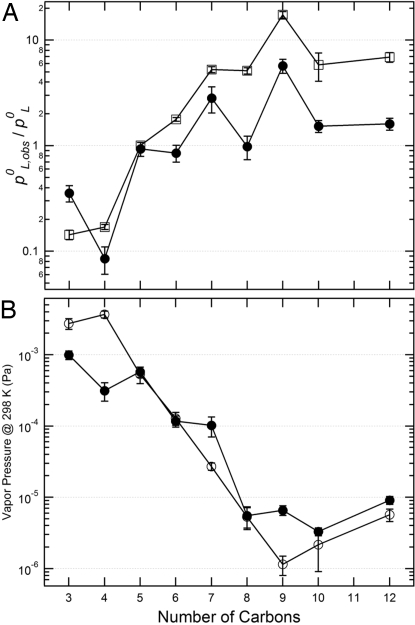
The specific deviations from ideal behavior were quantified by comparing the observed vapor pressures to those of the pure compound vapor pressures (Fig. 2A). In general, the ratios of the vapor pressures of the observed and pure compounds, which are equivalent to the activity coefficients in this “organic solution” (γi = pL,obs0/pL0), were <1 for the lower-molecular-weight diacids but >1 for the heavier diacids. In the case of the lowest-molecular-weight diacids, the observed vapor pressures were ≈3–10 times lower than for an ideal solution; consequently, partitioning of these compounds to the particle phase will be greater than expected based on the pure component vapor pressures. Whereas the liquid-like behavior of the mixtures derives from melting point depression, the deviations of the γi's from unity are effectively related to changes in the boiling point. A slight “odd-even” dependence of the pL,obs0 with respect to the diacid carbon number was observed (Fig. 2B). Such a dependence has been observed for the solid-phase vapor pressures of these compounds (12, 23, 24), but is somewhat surprising here given the clear liquid-like behavior of the mixture and lack of such behavior for the pL0.
Fig. 2.
Measured properties of the dicarboxylic acids in the multicomponent mixtures. (A) Activity coefficients (pL0/pL,obs0) at 313 K as a function of the number of carbon atoms comprising the diacid for the purely organic mixture (●) and the organic/inorganic mixture (□). Note the log y scale. Error bars are 1σ from the average of 3 independent measurements. (B) Effective vapor pressures for the diacids in the 9-component purely organic mixture (●) and for the pure liquids (○) at 298 K.
The measured activity coefficients for the individual diacids in the multicomponent mixture were observed to be quite variable even though they are chemically very similar and differ only in the number of –CH2 groups (Fig. 2A). Nonunity activity coefficients for aerosol components have been previously inferred from other measurements (25, 26); however, it has generally been asserted that nonunity γ's arise from mixing together very dissimilar materials and are usually >1. The observation of activity coefficients that are less than unity is unexpected and therefore of particular interest, especially because it will lead to increased partitioning of these compounds to the particle phase. We note that only the smallest diacids (≈C3–C5) truly evaporate from a 9-component mixture because the composition of the sample constantly changes due to preferential evaporation of the higher-volatility compounds. The lower-volatility compounds evaporate from a mixture composed of increasingly fewer compounds, which may be the reason why the measured activity coefficients for the higher-molecular-weight compounds are generally closer to unity, although the observed desorption profiles indicate that the samples remained liquid-like over an entire TPD measurement.
We have compared our measured activity coefficients to predictions from the UNIFAC group-contribution method (22), which is commonly used to estimate vapor pressures and activity coefficients of organic aerosol components for use in OA modeling (25, 27, 28). In contrast to our observations, UNIFAC predicts 2 > γi ≥ 1 for each of the compounds in a 9-component diacid mixture. These results therefore suggest that the total interactions between the diacid molecules are currently not adequately represented as sums of parameterized binary interaction parameters between the functional groups of the molecules, in this case –COOH and –CH2 groups. Further development of estimation methods that consider other interactions, e.g., ternary interactions, may be necessary to accurately represent vapor pressures in models.
Our observed changes in volatility are unlikely to arise from condensed phase chemical reactions, such as esterification or dimerization (29), for the following 2 reasons: (i) If such reactions had occurred to any significant extent, the products should have been detectable unless they reverted back to the original diacids as the species evaporated. (ii) Condensed-phase chemical reactions seem unlikely because they are slow in the absence of a catalyst (e.g., a strong acid or base or an oxidant such as OH) and should not have occurred on the timescale of our measurements
Most atmospheric aerosols are actually mixtures of organic and inorganic compounds (2). Therefore, the influence of an inorganic salt (NaNO3) on the evaporation behavior of the mixtures was assessed for an equimolar total organic/inorganic mixture, where the organic fraction was composed of the same 9 diacids considered above (Fig. 1B). Interestingly, clear liquid-like behavior was still observed even when half of the sample was a nonvolatile inorganic salt. Importantly, the shapes of the desorption profiles provide strong evidence that the organic and inorganic fractions were internally mixed and did not separate into 2 distinct phases as the distillation proceeded. This is indicated by the relatively gradual falloff of the signals on the high-temperature side of the desorption profiles compared with the rapid falloff in the purely organic mixture. The observed changes in the shapes of the desorption profiles on addition of a nonvolatile component are qualitatively predicted by the evaporation model (Fig. 1B). On a side, we note that our observations suggest that atmospheric aerosols containing inorganic and organic compounds at low relative humidity (so called dry aerosols) may be internally mixed. However, the extent to which the inorganic and organic fractions mix may depend on the specific chemical nature of the organics present, and thus may lead to a fractionation of the various atmospheric components within individual particles. Internal mixing of the inorganic and organic compounds would affect the crystallization behavior of the inorganic components, which would have consequences for the hygroscopic properties of internally mixed organic/inorganic particles, in particular, with respect to their deliquescence and efflorescence behavior (30).
In addition, the presence of the inorganic salt led to a slight increase in the measured activity coefficients for many of the diacids, most noticeably for the largest compounds with low vapor pressures (Fig. 2A). However, the effective vapor pressure for the C3 diacid was actually reduced further, with γmalonic decreased by more than a factor of 2 from the purely organic mixture. Evidently, the addition of an inorganic salt influences the nature of the intermolecular interactions in compound-specific ways. As with the purely organic mixture, these results suggest that group contribution methods may only have limited success in accurately predicting activity coefficients in complex nonaqueous mixtures.
It is possible that the mixture was not “liquid-like” throughout the entire sample, but instead contained distinct solid and liquid organic phases, with the liquid phase being a nonaqueous solution of the diacids in equilibrium with the solid phase, i.e., a “nonaqueous phase liquid” (e.g., ref. 26). However, the observation of continuous distillation of the individual diacids from the mixture indicates that this is not the case (see SI Text). Our observations are therefore more consistent with the 9-component diacid mixture being a well-mixed liquid rather than being composed of distinct solid and liquid organic phases.
These observations cannot directly establish whether the samples are supercooled liquids (metastable) or are what are termed “subcooled” liquids (thermodynamically stable), only that their evaporation behavior indicates that the samples were well-mixed, and therefore liquid-like. (A subcooled liquid is just a special case of nonaqueous phase liquids where the melting point depression is sufficiently large that there is no solid phase present.) The physical driving force behind the liquid-like behavior of the mixture of solid compounds is melting point depression. Melting point depression is primarily an entropic effect and can be estimated for each compound (assuming ideal behavior) from
where Tm,i is the melting point and ΔSfus,i is the entropy of fusion of the pure compound, R is the ideal gas constant, and Tmix,i is the melting point of compound i in a mixture with mole fraction xi (18). For the mixture considered here, succinic acid (C4) has the highest melting point both as a pure compound and in the ideal mixture. Tmix for succinic acid is reduced to 366 K in the 9-component mixture, which is at the high end of the temperature range over which the measurements were made. This therefore suggests that the mixture was supercooled, as opposed to subcooled.
To explore this further, we have qualitatively investigated the melting and freezing behavior of a macroscopic sample of this 9-component mixture. At room temperature, the mixture had a white, waxy appearance. On heating, the mixture became increasingly more liquid-like, as evidenced by the sample becoming more translucent. The sample turned clear in its entirety around ≈360–370 K, indicating that it had melted completely, generally consistent with the expected melting point depression (18). On cooling, the liquid sample could be readily supercooled up to ≈40 K below this “melting” temperature before turning opaque. This raises the possibility that atmospheric aerosols can exhibit liquid-like properties because they are supercooled, and not subcooled. Because the ability to nucleate and crystallize into a solid will vary as a cube of the particle diameter, atmospheric aerosols can remain liquids much longer than our bulk substrates. From the TPD experiments we deduced that the samples remain liquid-like for at least a few hours. Given the differences in volume, we estimate that the atmospheric organic aerosols can stay as liquids for days, unless they have a nucleating agent embedded in them.
Supercooling of organic aerosol is not unprecedented, and it has also recently been invoked to describe the chemical reactivity of a 2-component system (where only one of the components was a thermodynamically stable liquid) (31). Additionally, the tendency of binary and ternary mixtures of polycyclic aromatic hydrocarbons, for example, to form supercooled liquids during phase behavior studies has been reported (32). However, atmospheric organic aerosols typically comprise hundreds or thousands of different compounds, and the melting point depression will consequently be larger than for any of these systems, including the 9-component mixture considered here (18). In any case, identification of the liquid-like behavior is all that is needed for dealing with gas-particle partitioning in atmospheric modeling calculations (19) and in understanding whether multiphase reactions are surface or volume limited (33).
Studies involving a larger number of atmospheric organic aerosol constituents are needed to place our observations of vapor pressure variations and liquid-like behavior on firmer footing. Such studies will greatly enhance implications of the role of nonidealities on OA formation and modeling (25, 27, 28, 34, 35). Yet, we can note the implications of our findings to the atmosphere with the assumptions that our observations are applicable to all atmospheric organic aerosols and their components. Models that use detailed chemical mechanisms to describe the gas-particle partitioning tend to give poor agreement with both laboratory studies and field observations (8, 35–38). These simulations of aerosol formation therefore often “adjust” the vapor pressures of the semivolatile organic compounds to achieve better agreement with observations (8, 35–39). Such adjustments universally lower the vapor pressures. Partitioning theory indicates that actual and predicted organic aerosol abundances are most strongly influenced by nonideal behavior of compounds with saturation vapor pressures that are close to the actual organic aerosol loading (within approximately a factor of 10) (19, 40). For typical ambient organic aerosol loadings [≈5 μg/m3 (3)], deviations from ideality for compounds with p0 values that range from ≈10−5 Pa to 10−3 Pa will contribute most importantly to variability in observed atmospheric organic aerosol loadings. In laboratory studies, where the organic loading is often much larger, this sensitive range of p0 values will be shifted to higher values. Because the pL0 of the C3-C7 diacids considered here are close to ambient loadings (Fig. 2B), the nonideal behavior of these compounds will have a significant impact on their gas-particle partitioning, much more so than for the larger, lower vapor pressure compounds. Our observations that the vapor pressures of the more abundant, lower-molecular-weight components in organic mixtures are lower than those of the pure compounds (i.e., γi < 1) will thus lead to better agreements between calculations and observations with less need for “adjustments.”
Our results provide important experimental support for a conceptual framework in which activity coefficients of partitioned compounds may differ significantly from unity. This can occur in complex mixtures of similar compounds, and may therefore influence OA formation both in the laboratory and the atmosphere. Last, we note that our experimental determination that the mixtures of organics exhibit liquid-like behavior provides an important justification for the use of the gas-liquid partitioning model.
Methods Summary
A temperature-programmed desorption method (11, 12) was used to measure evaporation rates of the C3–C10 and C12 straight-chain diacarboxylic acids from equimolar mixtures of the diacids (see SI Text for further details). Proton transfer reaction mass spectrometry (PT-RMS) was used to monitor the gas-phase signals due to evaporating molecules as the temperature of the sample was linearly increased during each TPD experiment. The samples used in the TPD analysis were formed by deposition of aerosols that were generated by atomization of methanol solutions of the diacids. The methanol was removed from the aerosols before deposition by passing them through a diffusion drier.
The observed temperature-dependent evaporation rates were compared with a multicomponent evaporation model. In the model, the mixture was assumed to be well mixed and the thermodynamic properties of each component (p0(T), ΔHvap and ΔSvap) were specified individually. Evaporation rates for each component were determined by using the Hertz–Knudsen relationship and the mole fraction of each compound.
Supplementary Material
Acknowledgments.
We thank T. Thornberry, J. de Gouw, J. Burkholder and J. Kroll for useful discussions. This work was supported by the National Oceanic and Atmospheric Administration program for climate change.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802144105/DCSupplemental.
References
- 1.Intergovernmental Panel on Climate Change (IPCC) Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge, UK: Cambridge Univ Press; 2007. [Google Scholar]
- 2.Murphy DM. Something in the air. Science. 2005;307:1888–1890. doi: 10.1126/science.1108160. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, et al. Ubiquity and dominance of oxygenated species in organic aerosols in anthropogenically-influenced Northern Hemisphere midlatitudes. Geophys Res Lett. 2007 doi: 10.1029/2007GL029979. [DOI] [Google Scholar]
- 4.Seinfeld JH, Pankow JF. Organic atmospheric particulate material. Annu Rev Phys Chem. 2003;54:121–140. doi: 10.1146/annurev.physchem.54.011002.103756. [DOI] [PubMed] [Google Scholar]
- 5.de Gouw JA, et al. Budget of organic carbon in a polluted atmosphere: Results from the New England Air Quality Study in 2002. J Geophys Res-Atmos. 2005 16310.11029/12004JD005623. [Google Scholar]
- 6.Heald CL, et al. A large organic aerosol source in the free troposphere missing from current models. Geophys Res Lett. 2005 18810.11029/12005GL023831. [Google Scholar]
- 7.Volkamer R, et al. Secondary organic aerosol formation from anthropogenic air pollution: Rapid and higher than expected. Geophys Res Lett. 2006 17810.11029/12006GL026899. [Google Scholar]
- 8.Johnson D, et al. Simulating regional scale secondary organic aerosol formation during the TORCH 2003 campaign in the southern UK. Atmos Chem Phys. 2006;6:403–418. [Google Scholar]
- 9.Robinson AL, et al. Rethinking organic aerosols: Semivolatile emissions and photochemical aging. Science. 2007;315:1259–1262. doi: 10.1126/science.1133061. [DOI] [PubMed] [Google Scholar]
- 10.McFiggans G, et al. The effect of physical and chemical aerosol properties on warm cloud droplet activation. Atmos Chem Phys. 2006;6:2593–2649. [Google Scholar]
- 11.Cappa CD, Lovejoy ER, Ravishankara AR. Evaporation rates and vapor pressures of the even numbered C8-C18 monocarboxylic acids. J Phys Chem A. 2008;112:3959–3964. doi: 10.1021/jp710586m. [DOI] [PubMed] [Google Scholar]
- 12.Cappa CD, Lovejoy ER, Ravishankara AR. Determination of evaporation rates and vapor pressures of very low volatility compounds: A study of the C4–C10 and C12 dicarboxylic acids. J Phys Chem A. 2007;111:3099–3109. doi: 10.1021/jp068686q. [DOI] [PubMed] [Google Scholar]
- 13.Li YC, Yu JZ. Simultaneous determination of mono- and dicarboxylic acids, omega-oxo-carboxylic acids, midchain ketocarboxylic acids, and aldehydes in atmospheric aerosol samples. Environ Sci Technol. 2005;39:7616–7624. doi: 10.1021/es050896d. [DOI] [PubMed] [Google Scholar]
- 14.Varutbangkul V, et al. Hygroscopicity of secondary organic aerosols formed by oxidation of cycloalkenes, monoterpenes, sesquiterpenes, and related compounds. Atmos Chem Phys. 2006;6:2367–2388. [Google Scholar]
- 15.Lide DR, editor. CRC Handbook of Chemistry and Physics. Boca Raton, FL: Taylor & Francis; 2007. [Google Scholar]
- 16.Gong HM, Matsunaga A, Ziemann PJ. Products and mechanism of secondary organic aerosol formation from reactions of linear alkenes with NO3 radicals. J Phys Chem A. 2005;109:4312–4324. doi: 10.1021/jp058024l. [DOI] [PubMed] [Google Scholar]
- 17.Lim YB, Ziemann PJ. Products and mechanism of secondary organic aerosol formation from reactions of n-alkanes with OH radicals in the presence of NOx. Environ Sci Technol. 2005;39:9229–9236. doi: 10.1021/es051447g. [DOI] [PubMed] [Google Scholar]
- 18.Marcolli C, Luo BP, Peter T. Mixing of the organic aerosol fractions: Liquids as the thermodynamically stable phases. J Phys Chem A. 2004;108:2216–2224. [Google Scholar]
- 19.Pankow JF. An absorption-model of the gas/aerosol partitioning involved in the formation of secondary organic aerosol. Atmos Environ. 1994;28:189–193. [Google Scholar]
- 20.Roux MV, Temprado M, Chickos JS. Vaporization, fusion and sublimation enthalpies of the dicarboxylic acids from C-4 to C-14 and C-16. J Chem Thermodyn. 2005;37:941–953. [Google Scholar]
- 21.Hansen AR, Beyer KD. Experimentally determined thermochemical properties of the malonic acid/water system: Implications for atmospheric aerosols. J Phys Chem A. 2004;108:3457–3466. [Google Scholar]
- 22.Reid RC, Prausnitz JM, Poling BE. The Properties of Gases and Liquids. New York: McGraw–Hill; 1987. [Google Scholar]
- 23.Bilde M, Svenningsson B, Monster J, Rosenorn T. Even-odd alternation of evaporation rates and vapor pressures of C3–C9 dicarboxylic acid aerosols. Environ Sci Technol. 2003;37:1371–1378. [Google Scholar]
- 24.Chattopadhyay S, Ziemann PJ. Vapor pressures of substituted and unsubstituted monocarboxylic and dicarboxylic acids measured using an improved thermal desorption particle beam mass spectrometry method. Aerosol Sci Technol. 2005;39:1085–1100. [Google Scholar]
- 25.Jang M, Kamens RM, Leach KB, Strommen MR. A thermodynamic approach using group contribution methods to model the partitioning of semivolatile organic compounds on atmospheric particulate matter. Environ Sci Technol. 1997;31:2805–2811. [Google Scholar]
- 26.Mukherji S, Peters CA, Weber WJ. Mass transfer of polynuclear aromatic hydrocarbons from complex DNAPL mixtures. Environ Sci Technol. 1997;31:416–423. [Google Scholar]
- 27.Pankow JF, Seinfeld JH, Asher WE, Erdakos GB. Modeling the formation of secondary organic aerosol. 1. Application of theoretical principles to measurements obtained in the α-pinene/, β- pinene/, sabinene/, Δ(3)-carene/, and cyclohexene/ozone systems. Environ Sci Technol. 2001;35:1164–1172. doi: 10.1021/es001321d. [DOI] [PubMed] [Google Scholar]
- 28.Bowman FM, Melton JA. Effect of activity coefficient models on predictions of secondary organic aerosol partitioning. J Aerosol Sci. 2004;35:1415–1438. [Google Scholar]
- 29.Rissman TA, et al. Cloud condensation nucleus (CCN) behavior of organic aerosol particles generated by atomization of water and methanol solutions. Atmos Chem Phys. 2007;7:2949–2971. [Google Scholar]
- 30.Marcolli C, Krieger UK. Phase changes during hygroscopic cycles of mixed organic/inorganic model systems of tropospheric aerosols. J Phys Chem A. 2006;110:1881–1893. doi: 10.1021/jp0556759. [DOI] [PubMed] [Google Scholar]
- 31.Hearn JD, Smith GD. Measuring rates of reaction in supercooled organic particles with implications for atmospheric aerosol. Phys Chem Chem Phys. 2005;7:2549–2551. doi: 10.1039/b506424d. [DOI] [PubMed] [Google Scholar]
- 32.Peters CA, Mukherji S, Knightes CD, Weber WJ. Phase stability of multicomponent NAPLs containing PAHs. Environ Sci Technol. 1997;31:2540–2546. [Google Scholar]
- 33.Ravishankara AR. Heterogeneous and multiphase chemistry in the troposphere. Science. 1997;276:1058–1065. [Google Scholar]
- 34.Camredon M, Aumont B, Lee-Taylor J, Madronich S. The SOA/VOC/NOx system: An explicit model of secondary organic aerosol formation. Atmos Chem Phys. 2007;7:5599–5610. [Google Scholar]
- 35.Jenkin ME. Modelling the formation and composition of secondary organic aerosol from alpha- and beta-pinene ozonolysis using MCM v3. Atmos Chem Phys. 2004;4:1741–1757. [Google Scholar]
- 36.Colville CJ, Griffin RJ. The roles of individual oxidants in secondary organic aerosol formation from Delta(3)-carene: 2. SOA formation and oxidant contribution. Atmos Environ. 2004;38:4013–4023. [Google Scholar]
- 37.Johnson D, Jenkin ME, Wirtz K, Martin-Reviejo M. Simulating the formation of secondary organic aerosol from the photooxidation of toluene. Environ Chem. 2004;1:150–165. [Google Scholar]
- 38.Johnson D, Jenkin ME, Wirtz K, Martin-Reviejo M. Simulating the formation of secondary organic aerosol from the photooxidation of aromatic hydrocarbons. Environ Chem. 2005;2:35–48. [Google Scholar]
- 39.Griffin RJ, Dabdub D, Seinfeld JH. Development and initial evaluation of a dynamic species-resolved model for gas phase chemistry and size-resolved gas/particle partitioning associated with secondary organic aerosol formation. J Geophys Res-Atmos. 2005 05310.01029/02004JD005219. [Google Scholar]
- 40.Donahue NM, Robinson AL, Stanier CO, Pandis SN. Coupled partitioning, dilution, and chemical aging of semivolatile organics. Environ Sci Technol. 2006;40:2635–2643. doi: 10.1021/es052297c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.