Abstract
Multiple sclerosis (MS) is a chronic relapsing disease of the central nervous system (CNS) in which immune processes are believed to play a major role. To date, there is no reliable method by which to characterize the immune processes and their changes associated with different forms of MS and disease progression. We performed antigen microarray analysis to characterize patterns of antibody reactivity in MS serum against a panel of CNS protein and lipid autoantigens and heat shock proteins. Informatic analysis consisted of a training set that was validated on a blinded test set. The results were further validated on an independent cohort of relapsing–remitting (RRMS) samples. We found unique autoantibody patterns that distinguished RRMS, secondary progressive (SPMS), and primary progressive (PPMS) MS from both healthy controls and other neurologic or autoimmune driven diseases including Alzheimer's disease, adrenoleukodystropy, and lupus erythematosus. RRMS was characterized by autoantibodies to heat shock proteins that were not observed in PPMS or SPMS. In addition, RRMS, SPMS, and PPMS were characterized by unique patterns of reactivity to CNS antigens. Furthermore, we examined sera from patients with different immunopathologic patterns of MS as determined by brain biopsy, and we identified unique antibody patterns to lipids and CNS-derived peptides that were linked to each type of pathology. The demonstration of unique serum immune signatures linked to different stages and pathologic processes in MS provides an avenue to monitor MS and to characterize immunopathogenic mechanisms and therapeutic targets in the disease.
Keywords: antibodies, autoimmunity, biomarker
Multiple sclerosis (MS) is a chronic inflammatory disease of the central nervous system (CNS) of presumed autoimmune etiology (1). Approximately 85–90% of patients begin with a relapsing–remitting (RRMS) course and 40% eventually become progressive (secondary progressive MS, SPMS); in 10%, MS presents a primary progressive course (PPMS). MS is also heterogeneous in its immunopathological patterns of active infiltrating inflammatory cells and demyelination (2).
There is evidence that immune processes play a major role in disease pathogenesis and progression (1); however, to date there has been no reliable method by which to identify and characterize immune processes in the serum that are unique to MS. Antigen microarrays are newly developed tools for the high-throughput characterization of the immune response (3), and have been used to analyze immune responses in vaccination and in autoimmune disorders (3–6). Our interest in investigating autoreactivity to arrays of self-antigens in MS is based on the hypothesis that patterns of multiple reactivities may be more revealing than single antigen–antibody relationships (7, 8) as shown in our previous analysis of autoimmune repertoires of mice (5, 9) and humans (10, 11) in health and disease. Thus, autoantibody repertoires have the potential to provide insights into the pathogenesis of the disease and to serve as immune biomarkers (12) of the disease process.
Results
Conditions to Detect Specific Microarray Autoantibodies in MS.
We constructed antigen microarrays using 362 myelin and inflammation-related antigens [supporting information (SI) Table S1] that encompassed CNS antigens associated with MS, CNS antigens associated with other neurological diseases and heat shock proteins (HSP). Antigens were spotted on epoxy glass slides as described (5).
We compared the sensitivity of the antigen-microarray technique with that of a standard ELISA technique using commercially available monoclonal and polyclonal antibodies directed against CNS, HSP and lipid antigens. The antigen microarray detected antigen reactivities at log10 dilutions that were 1–2 logs greater than the reactivities detected by using the ELISA method (Table S2). Thus, the antigen microarray appear to be more sensitive than a standard ELISA. These results are consistent with published reports (3).
To determine which serum dilution was optimal to investigate immune signatures in MS, we analyzed the reactivity of healthy controls (HC) and RRMS subjects at dilutions of 1:10, 1:100 and 1:1,000 for both IgG and IgM antibodies. In MS the mean IgG antibody reactivity to CNS antigens, lipids and heat shock proteins was highest at 1:10 as compared with 1:100 and 1:1,000 where minimal reactivity was observed (P < 0.0001, 2-way ANOVA, Fig. S1A). The mean IgG reactivity was also highest at a 1:10 dilution in HC (P < 0.0001, 2-way ANOVA), but this reactivity was less than that manifested in MS subjects (P < 0.001, P < 0.001 and P < 0.05 for CNS antigens, lipids and heat shock proteins respectively, 2-way ANOVA); indeed, at dilutions of 1:100 and 1:1,000, there were no differences between the magnitude of IgG reactivity in MS compared with HC. The IgM reactivities in controls were as high, if not higher than in MS subjects. This is consistent with the observation that healthy humans are born with IgM autoantibodies to myelin antigens and heat shock proteins (11). Because MS subjects manifested significantly elevated serum IgG autoantibodies at a 1:10 dilution, we investigated serum antibody patterns with antigen microarrays by using this dilution.
To establish that the reactivity detected at a 1:10 dilution was specific, we carried out inhibition experiments that demonstrated that reactivity to PLP261–277 on the antigen array could be inhibited by preincubation of the serum with excess, unbound PLP261–277, but not with a control peptide, HSP601–20 (Fig. S1B).
Autoantibody Pattern Analysis Identifies an Immune Signature for RRMS.
To investigate whether we could identify unique antibody signatures in RRMS we studied the antibody repertoire in 38 patients with RRMS and 30 HC subjects. We allocated samples into a training set (24 RRMS and 20 controls) and a randomly selected test set (14 RRMS and 10 controls). The training set was used to determine whether we could identify patterns of antibody reactivity that could discriminate RRMS from control samples. If such patterns were found, they were then validated on the test set. The training set was analyzed by using the Wilcoxon–Mann–Whitney test; we controlled for the false discovery rate using the method of Benjamini and Hochberg (13). The clinical characteristics of the patients and HC are listed in Table S3.
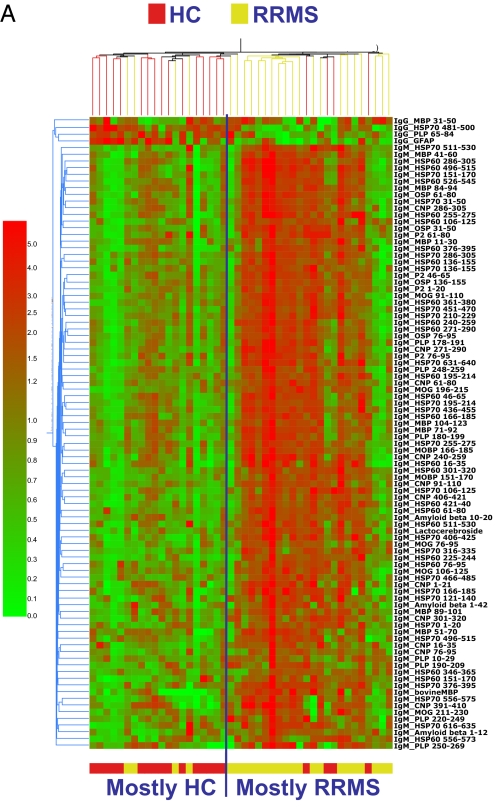
As shown in the heat map in Fig. 1A, we identified a pattern of reactivity that distinguished RRMS from HC (P < 0.0001, Fisher's exact test). This pattern consisted of 94 antibody reactivities (Table S4). Of the 94 reactivities, 90 were up-regulated and 4 were down-regulated in MS versus controls. Thus, RRMS was associated with both a gain and a loss of particular autoreactivities. Of the up-regulated reactivities, 50% were IgM antibodies binding to peptides of CNS antigens and 49% were IgM antibodies binding to peptides of heat shock proteins. The ability to distinguish MS vs. controls was not observed at dilutions of 1:100 or 1:1,000 (data not shown).
Fig. 1.
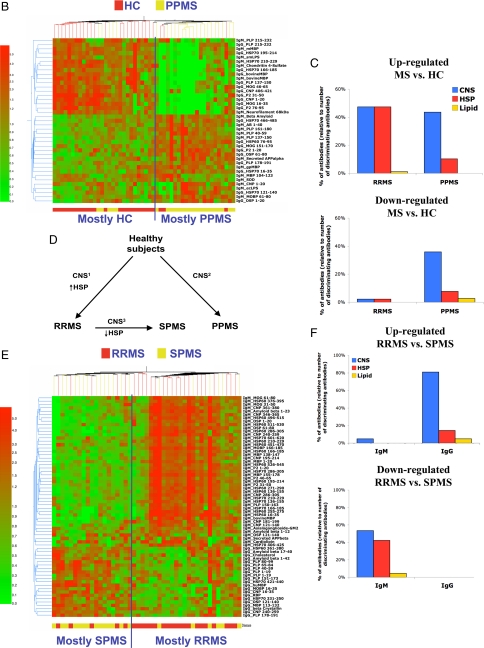
Serum antibody reactivity in RRMS and PPMS. (A and B) Antibody reactivities discriminating RRMS (A) and PPMS (B). Shown is a heat map depicting the antibody reactivity in RRMS (A), PPMS (B) or HC samples. The antibody reactivities included in these heat maps are listed in Table S4 (RRMS) and Table S5 (PPMS). (C) Antigen specificity in RRMS and PPMS, shown as the relative contribution of CNS, HSP, and lipid antigens (percentage relative to total number of discriminating antigens) found to be up- or down-regulated in MS relative to HC. (D) Diagram summarizing the immune signatures associated with RRMS, SPMS, and PPMS. (E) Heat map depicting the antibody reactivities in SPMS and RRMS samples. The antibody reactivities included in this heat map are listed in Table S8. (F) Antigen specificity in SPMS, shown as the relative contribution of CNS, HSP, and lipid antigens (percentage relative to total number of discriminating antigens) found to be up- or down-regulated in SPMS relative to RRMS.
To validate the discriminating pattern shown in Fig. 1A, we performed a leave-1-out cross-validation analysis (LOOCV) in the training set (13) and then validated the results on the test set. For the LOOCV in the training set, the number of true (correct) and false (incorrect) classifications was computed to estimate the success rate, positive predictive value (PPV), negative predictive value (NPV) in the training set. The LOOCV revealed a positive predictive value (PPV)—defined as the fraction of RRMS patients identified as RRMS by their antigen microarray reactivity—of 0.75 and a negative predictive value (NPV)—defined as the fraction of HC identified as HC by their antigen microarray reactivity—of 0.90; the success rate was 0.83 (P < 0.0001). The most rigorous validation is to test the patterns identified in the training set to determine whether they can differentiate MS subjects from HC in the test set. We found that the pattern identified in the training set was able to classify the test set of samples with a PPV of 0.85 and a NPV of 0.80, and with an success rate of 0.83 (P = 0.004, Fisher's exact test).
To further validate our findings, we analyzed 51 untreated RRMS obtained from the University of Seville to determine whether we could distinguish RRMS from HC using an independent cohort of samples from another institution and geographic area. We were able to discriminate RRMS from HC in this independent cohort with a success rate of 0.69 with a PPV of 0.73 and a NPV of 0.58 (P = 0.01, Fisher's exact test).
As a specificity control for the patterns detected in MS, we investigated sera from patients with systemic lupus erythematosus (SLE), adrenoleukodystrophy (ALD) and Alzheimer's disease (AD). SLE is a chronic autoimmune disease characterized by circulating antibodies to a broad range of self-antigens (14). ALD is a degenerative disorder characterized by the accumulation of very long-chain fatty acids and a CNS neuroinflammatory process that shares features with MS (15). AD is not considered an autoimmune disease; however, immune responses to β-amyloid-derived peptides have been reported (reviewed in ref. 16). We found that antibody patterns detected on antigen microarrays discriminated RRMS from SLE, ALD and AD samples (P < 0.0001, Fisher's exact test).
Autoantibody Pattern Analysis Identifies an Immune Signature for PPMS.
PPMS has a different clinical course than RRMS, and it has been suggested that PPMS may involve disease mechanisms different from those in RRMS (17). We studied 24 PPMS and 25 age- and gender- matched HC in a training set, and 13 PPMS and 12 controls in a test set of samples. The heat map in Fig. 1b shows the antibody reactivities that passed significance tests and could discriminate PPMS and HC both in the training set (P < 0.0001, Fisher's exact test) and the test set (P < 0.01, Fisher's exact test). The LOOCV on the learning set revealed an overall efficiency of 86%, with PPV = 0.87 and NPV = 0.85. The efficiency for the test set was 72%; the PPV = 0.79 and the NPV = 0.75. As with RRMS, antigen microarrays were able to discriminate PPMS from control subjects at a 1:10 dilution but not at dilutions of 1:100 or 1:1,000. Furthermore, as with RRMS and SPMS, the antigen microarray analysis discriminated between PPMS and other diseases (SLE, ALD, AD; P < 0.001, Fisher's exact test).
The discriminating reactivities in PPMS were IgG (51%) and IgM (49%) and were mainly directed against CNS antigens (Fig. 1 B and C and Table S5). The CNS antigens in the PPMS immune signature were different from those in the RRMS signature. We have termed the RRMS CNS signature CNS1 and the PPMS CNS signature CNS2 (Fig. 1D and Table S6). When we further compared RRMS and PPMS we found a pronounced reactivity against HSP60 or HSP70 in RRMS that was not observed in PPMS (Fig. 1 A–D and Table S7). Furthermore, 46% of the discriminating reactivities in PPMS consisted of antibodies that were decreased in PPMS compared with HC, whereas in RRMS only 4% of the discriminating antibodies were decreased compared with HC (Figs. 1 B and C). There was only a minor overlap between the reactivities that discriminated PPMS and those that discriminated RRMS compared with HC (Table S6 and Table S7). This finding is compatible with the view that different immune processes occur in these 2 forms of MS (17).
Autoantibody Pattern Analysis Identifies an Immune Signature for SPMS.
Approximately 50% of the RRMS patients become progressive (SPMS) (18). Although there is no consensus on the mechanisms involved in the transition to SPMS, several studies suggest changes in the nature of the inflammatory response and the emergence of neurodegenerative processes occur in the secondary progressive phase of MS (18). Having identified an autoantibody signature in RRMS that consisted of increased reactivity to HSP and a unique pattern of reactivity to CNS antigens (CNS1) we studied the antibody signature associated with SPMS by comparing antibody reactivity in 37 RRMS vs. 30 SPMS samples (Fig. 1E).
We found that SPMS could be discriminated from RRMS with a success rate of 71% (P = 0.0073). SPMS was characterized by a decrease in the IgM antibodies to HSP60 and HSP70 that we found in RRMS (Table S7 and Table S8 and Fig. 1E). Thus, SPMS and PPMS are similar in that both have only minimal reactivity to HSP. When we examined the CNS reactivity in SPMS we found a decrease in CNS IgM antibodies that were up-regulated in RRMS, and an increase in CNS-reactive IgG antibodies. The CNS signature for SPMS differed from both RRMS and PPMS and we have termed it CNS3 (Figs. 1 D–F and Table S6).
Autoantibody Patterns Distinguish Pathologic Subtypes of MS.
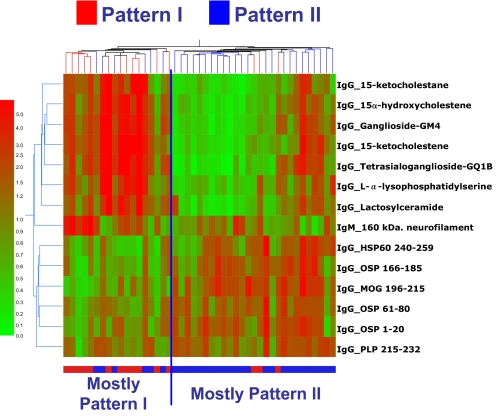
Lucchinetti, Bruck and Lassman have defined four immunopathologic patterns of MS (2). The pattern of active demyelination is identical among multiple active lesions examined from a given MS patient, yet heterogeneous between patients, suggesting pathogenic heterogeneity. Pattern I is characterized by T cell/macrophage-mediated demyelination. Pattern II is characterized by antibody/complement-associated demyelination. Pattern III is defined by a distal oligodendrogliopathy, and pattern IV is characterized by oligodendrocyte degeneration in the periplaque white matter; to date pattern IV has only been identified in autopsy cases. Patterns I and II lesions show the typical perivenous distribution and sharp borders that are the pathological hallmarks of MS lesions and are thought to result from classical autoimmune mechanisms (2). We investigated serum taken at the time of brain biopsy from 15 Pattern I and 30 Pattern II subjects. As shown in Fig. 2, we were able to discriminate pattern I from pattern II (P = 0.0082, Fisher's exact test). To validate this finding, we analyzed a blinded set of samples that contained the 15 pattern I that we used for analysis above mixed randomly with 23 new pattern II samples. In this validation test, we were also able to distinguish pattern I from pattern II (P = 0.0017, Fisher's exact test). The LOOCV on the learning set revealed a success rate of 0.78, with PPV = 0.78 and NPV = 0.67, the success rate for the test set was 0.78; the PPV = 0.82 and the NPV = 0.73.
Fig. 2.
Antibody reactivity associated with brain pathology. Shown is a heat map depicting the antibody reactivity in Pattern I and Pattern II samples according to the colorimetric scale shown on the left. The antibody reactivities used to construct this heat map are listed in Table S9.
The immune signature that distinguished pattern I from pattern II consisted of 13 IgG and 1 IgM reactivities against lipids, HSP and CNS antigens (Fig. 2). Pattern II subjects showed increased IgG reactivity to HSP60, MOG, OSP and PLP peptide epitopes. Noteworthy, the up-regulated reactivities in pattern I subjects were IgG antibodies to 7 lipids; 3 of these lipids were oxidized derivatives of cholesterol (15-ketocholestene, 15-ketocholestane and 15a-hydroxycholestene).
Cholesterol Derivatives Worsen Experimental Autoimmune Encephalomyelitis (EAE).
To explore the relationship between autoantibodies to oxidized cholesterol derivatives (oxChol) and disease pathology, we examined the effect of these lipids on EAE, which serves as an immune model of MS. We administered 15-ketocholestene, 15-ketocholestane and 15α-hydroxycholestene to C57BL/6 mice at the time of EAE induction with MOG35–55 and on days 4, 7 and 10 after induction. Administration of oxChol enhanced EAE as measured clinically (Fig. S2A, P < 0.0001, 2-way ANOVA), and augmented inflammatory infiltrates (P < 0.05, 1-way ANOVA), demyelination (P < 0.01, 1-way ANOVA) and axonal loss (P < 0.001, 1-way ANOVA) (Fig. S2B).
It has been postulated that the oxidized derivative of cholesterol, 7-ketocholesterol, contributes to MS pathology by activating microglial cells via a poly (ADP-ribose)-polymerase-1 enzyme (PARP)-dependent pathway (19). To investigate whether the effect of oxChol on EAE we observed was mediated by PARP we used the PARP inhibitor, 5-Aminoisoquinolinone (AIQ) (20). We found that AIQ abrogated the worsening of EAE caused by oxChol both clinically (P < 0.0001, 2-way ANOVA) and histopathologically (P < 0.001, 1-way ANOVA) (Fig. S2) but did not affect T cell responses to MOG35–55 as measured by cytokines (IFN-γ and IL-17) or proliferation (data not shown). In addition, transfer of serum from oxChol-treated mice did not enhance EAE. Taken together, these results suggest that the effect of oxChol on EAE is due to the effect of oxChol through PARP and not through the induction of anti-lipid antibodies or affecting adaptive T cell responses to MOG35–55.
Discussion
We report here that antigen-microarray analysis of autoantibodies can identify serum autoantibody signatures associated with different clinical forms and pathologic subtypes of MS; the signatures were based on collective autoantibody patterns, not single autoantibody reactivities. These informative patterns emerged from autoantibodies that bound peptides of myelin molecules and HSP, proteins and lipids; the informative autoantibodies were detectable at 1:10, but not at higher dilutions. Moreover, the informative patterns included decreases as well as increases of autoantibody reactivities relative to those found in healthy subjects. Antigen microarrays have been used to characterize serum autoantibodies in SLE (21) and rheumatoid arthritis (22), but have not been successfully applied to MS. In the CSF, antigen microarrays in MS have detected antibodies to lipid (6) and αB-crystallin (23) and of note, the αB-crystallin reactive antibodies were of low affinity, detectable at 1:20 dilution (23). High-affinity autoreactive antibodies in the serum have not been consistently found in MS (24–28).
We found that unique autoantibody signatures characterize RRMS, SPMS and PPMS based on reactivity to CNS antigens and HSP. Each of the different clinical forms of MS had a unique antibody signature directed against CNS antigens that we have termed CNS1 (RRMS), CNS2 (PPMS) and CNS3 (PPMS). CNS1 was characterized by a broad IgM reactivity to CNS antigens, CNS2 by a more restricted IgM and IgG reactivity and CNS3 by an increase in IgG reactivity to CNS antigens. In addition to the reactivity to CNS antigens, RRMS was notable for a pronounced antibody response to HSP. Strikingly, antibody responses to HSP were markedly decreased in both SPMS and PPMS. HSP are augmented in response to inflammation, and HSP up-regulation has been reported in MS lesions (29). Thus, the decrease of reactivity to HSP in progressive forms of MS is consistent with the less inflammatory nature of progressive MS and its relative lack of response to immunomodulatory therapy (17, 18). Indeed, we have found increased serum levels of HSP60 and HSP70 in RRMS patients (F.J.Q. and H.L.W., unpublished work).
In addition, HSP-specific immunity (30–32) and HSP60 itself (33) have been reported to be immunoregulatory. Thus, it is also possible that the intermittent attacks and recovery that characterize RRMS reflect HSP-linked immunomodulation.
We found that unique serum antibody patterns were associated with different patterns of MS pathology. Pattern II MS pathology was associated with increased IgG antibodies to HSP60, OSP, MOG and PLP peptide epitopes, whereas increased antibody reactivity to lactosylceramide and l-α-lysophosphatidylserine was linked to pattern I, and these antibodies have been described in the CSF of MS patients (6). Pattern I serum samples also contained antibodies to oxidized cholesterol derivatives. Increased levels of 7-ketocholesterol, a related oxidized derivative of cholesterol, have been found in the CSF of MS patients and may contribute to MS pathology by activating microglial cells via a PARP-1 dependent pathway (19). Consistent with this, we found that the administration of oxidized cholesterol derivatives worsened EAE by activating PARP-1 in microglia and CNS macrophages.
Pattern I pathology in MS is considered to result from macrophage/microglia-mediated demyelination, whereas Pattern II is thought to involve complement activation and other antibody-dependent mechanisms (34). Our results support a pathogenic role for oxidized derivatives of cholesterol and identify PARP as a target for therapeutic intervention in pattern I MS pathology. Furthermore, from a clinical standpoint patients with pattern II, but not I, have been reported to respond to plasmapheresis (35). Thus, the antibody signatures we have identified may be useful for identifying patients that would be responsive to treatment with plasmapheresis.
Although cell-mediated immunity against myelin antigens is felt to play a major role in MS (1), B cells and autoantibodies also appear to contribute to disease pathogenesis (36). How do our results relate to the role antibodies vs. T cells in the MS disease process? Most of the CNS antigen-reactive autoantibodies detected with our antigen microarrays are directed at linear epitopes. Antibodies to linear epitopes in MBP and MOG have been purified from active CNS lesions of MS patients (37) and may trigger the deposition of complement and other disease-amplifying mechanisms (38). In this regard, we detected antibodies to linear epitopes in CNS antigens in serum samples from MS patients with pattern II lesions, which are characterized by antibody deposition and complement activation (2). In addition, it has been shown that in MS patients there is an overlap between linear B and T cell epitopes targeted on MBP (39). Thus, the serum autoantibodies to linear epitopes might reflect the specificity of an ongoing T cell response. Note, however, that low-affinity autoantibodies are present in healthy individuals, and changes in the reactivity of these autoantibodies have been described in several autoimmune disorders including MS (40, 41). It is now recognized that MS is a complex heterogeneous immunologic disease (1). We believe that the serum autoantibody immune signatures we have identified reflect this complex process, not a single autoantibody that plays a dominant role in the disease such as would be the case for antibodies to the acetylcholine receptor in myasthenia gravis (42). Thus, the differences in the fine antibody specificity between MS and HC are not likely to identify pathogenic antibodies causing MS.
Taken together, how do the immune signatures we have identified relate to the disease process? As described above, the reactivity to HSP in RRMS appears related to the more inflammatory nature of MS in the initial relapsing stages. We believe that the different antibody signatures against CNS antigens also reflect the ongoing inflammatory process in the brain due to the traffic of immune cells, antibodies and/or antigen between the brain and periphery (36, 43). This is supported by our finding of unique serum patterns linked to type I or type II pathology as measured by brain biopsy. We have also studied antibody reactivity in paired CSF and serum samples. Our data suggest unique CSF antibody patterns in MS compared with other neurologic diseases and a partial overlap of the antibody response in the CSF compared with serum in MS (F.J.Q., M.F.F., G.I., M.L., I.R.C., and H.L.W., unpublished work).
In summary, our findings provide an avenue for the study of immune mechanisms in MS. The demonstration that serum microarray antibody patterns are linked to disease stage and pathologic subtype suggests that they may be used to monitor disease progression and aid in decisions regarding therapy. Furthermore, because the antibody signature appears to reflect immune processes in the CNS, antigen arrays provide a tool to identify new immunopathogenic mechanisms and therapeutic targets.
Materials and Methods
Antigens, Antibodies, and ELISA.
The antigens used in the construction of antigen microarrays are listed in Table S1, they were purchased from Sigma, Abnova, Matreya, Avanti Polar Lipids, Calbiochem, Chemicon, GeneTex, Novus Biologicals, Assay Designs, ProSci, EMD Biosciences, Cayman Chemical, HyTest, Meridian Life Science, and Biodesign International. The peptides were synthesized at Harvard Medical School. The antibodies were purchased from Abcam, Matreya, Abnova, Calbiochem, and Jackson ImmunoResearch. ELISA was performed as described (11).
Samples.
Serum samples from untreated RRMS during clinical remission, PPMS patients or HC were collected at the Partners MS Center; the patients did not present with other autoimmune disorders. Paired CSF and serum samples were collected at the University Hospital, School of Medicine, University of Seville from RRMS patients and controls. Sixty-two patients with biopsy-proven CNS inflammatory demyelinating disease were identified from the CNS biopsy cases belonging to the MS Lesion Project (MSLP). The MSLP database consists of a unique collection of biopsy-proven CNS cases with detailed pathological, clinical, imaging and serological material (NMSS RG3184-B-3-02). Active demyelinating lesions were classified into either pattern I or II based on published criteria (2). The clinical characteristics of the patients, pathological cohorts and healthy controls are listed in Table S3.
Antigen Microarray Production, Development, and Data Analysis.
The antigens were spotted as described (5, 10). The microarrays were blocked for 1 h at 37 °C with 1% BSA, and incubated for 2 h at 37 °C with the sample in blocking buffer. The arrays were then washed and incubated for 45 min at 37 °C with a 1:500 dilution of goat anti-human IgG Cy3-conjugated and goat anti-human IgM Cy5-conjugated detection antibodies (Jackson ImmunoResearch). The data were analyzed with the nonparametric Wilcoxon–Mann–Whitney test, by using the Benjamini and Hochberg method with a false discovery rate of 0.05 or 0.2 (for immunopathology pattern I and II samples) (3). The samples were classified by using a support vector machine constructed by using the antibody reactivities identified to be discriminatory on the training set (13).
EAE Induction.
All experiments were carried out in accordance with guidelines prescribed by the Institutional Animal Care and Use Committee at Harvard Medical School. EAE was induced and scored as described (44). AIQ (Sigma) was administered daily at 3 mg/kg, i.p. Axonal loss and demyelination were assessed at day 19 after EAE induction (44).
Supplementary Material
Acknowledgments.
This work was supported by National MS Society Grants NMSS RG3185-B-3-02 (to C.F.L.) and PP1289 (to H.L.W.) and National Institute of Neurologic Disorders and Stroke Grant NS049577-02 (to C.F.L.). F.J.Q. is a recipient of a long-term fellowship from the Human Frontiers of Science Program Organization.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806310105/DCSupplemental.
References
- 1.McFarland HF, Martin R. Multiple sclerosis: A complicated picture of autoimmunity. Nat Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti C, et al. Heterogeneity of multiple sclerosis lesions: Implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Robinson WH, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 4.Robinson WH, et al. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat Biotechnol. 2003;21:1033–1039. doi: 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- 5.Quintana FJ, et al. Functional immunomics: Microarray analysis of IgG autoantibody repertoires predicts the future response of mice to induced diabetes. Proc Natl Acad Sci USA. 2004;2(101) Suppl:14615–14621. doi: 10.1073/pnas.0404848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanter JL, et al. Lipid microarrays identify key mediators of autoimmune brain inflammation. Nat Med. 2006;12:138–143. doi: 10.1038/nm1344. [DOI] [PubMed] [Google Scholar]
- 7.Cohen IR. Tending Adam's Garden: Evolving the Cognitive Immune Self. London: Academic; 2000. [Google Scholar]
- 8.Quintana FJ, Merbl Y, Sahar E, Domany E, Cohen IR. Antigen-chip technology for accessing global information about the state of the body. Lupus. 2006;15:428–430. doi: 10.1191/0961203306lu2328oa. [DOI] [PubMed] [Google Scholar]
- 9.Quintana FJ, Cohen IR. Autoantibody patterns in diabetes-prone NOD mice and in standard C57BL/6 mice. J Autoimmun. 2001;17:191–197. doi: 10.1006/jaut.2001.0544. [DOI] [PubMed] [Google Scholar]
- 10.Merbl Y, Zucker-Toledano M, Quintana FJ, Cohen IR. Newborn humans manifest autoantibodies to defined self molecules detected by antigen microarray informatics. J Clin Invest. 2007;117:712–718. doi: 10.1172/JCI29943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintana FJ, Getz G, Hed G, Domany E, Cohen IR. Cluster analysis of human autoantibody reactivities in health and in type 1 diabetes mellitus: A bio-informatic approach to immune complexity. J Autoimmun. 2003;21:65–75. doi: 10.1016/s0896-8411(03)00064-7. [DOI] [PubMed] [Google Scholar]
- 12.Cohen IR. Real and artificial immune systems: Computing the state of the body. Nat Rev Immunol. 2007;7:569–574. doi: 10.1038/nri2102. [DOI] [PubMed] [Google Scholar]
- 13.Stekel D. Microarray Bioinformatics. Cambridge, UK: Cambridge Univ Press; 2003. [Google Scholar]
- 14.D'Cruz DP, Khamashta MA, Hughes GR. Systemic lupus erythematosus. Lancet. 2007;369:587–596. doi: 10.1016/S0140-6736(07)60279-7. [DOI] [PubMed] [Google Scholar]
- 15.Hudspeth MP, Raymond GV. Immunopathogenesis of adrenoleukodystrophy: Current understanding. J Neuroimmunol. 2007;182:5–12. doi: 10.1016/j.jneuroim.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Weiner HL, Frenkel D. Immunology and immunotherapy of Alzheimer's disease. Nat Rev Immunol. 2006;6:404–416. doi: 10.1038/nri1843. [DOI] [PubMed] [Google Scholar]
- 17.Miller DH, Leary SM. Primary-progressive multiple sclerosis. Lancet Neurol. 2007;6:903–912. doi: 10.1016/S1474-4422(07)70243-0. [DOI] [PubMed] [Google Scholar]
- 18.Rovaris M, et al. Secondary progressive multiple sclerosis: Current knowledge and future challenges. Lancet Neurol. 2006;5:343–354. doi: 10.1016/S1474-4422(06)70410-0. [DOI] [PubMed] [Google Scholar]
- 19.Diestel A, et al. Activation of microglial poly(ADP-ribose)-polymerase-1 by cholesterol breakdown products during neuroinflammation: A link between demyelination and neuronal damage. J Exp Med. 2003;198:1729–1740. doi: 10.1084/jem.20030975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald MC, et al. Effects of 5-aminoisoquinolinone, a water-soluble, potent inhibitor of the activity of poly (ADP-ribose) polymerase on the organ injury and dysfunction caused by haemorrhagic shock. Br J Pharmacol. 2000;130:843–850. doi: 10.1038/sj.bjp.0703391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li QZ, et al. Identification of autoantibody clusters that best predict lupus disease activity using glomerular proteome arrays. J Clin Invest. 2005;115:3428–3439. doi: 10.1172/JCI23587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hueber W, et al. Antigen microarray profiling of autoantibodies in rheumatoid arthritis. Arthritis Rheum. 2005;52:2645–2655. doi: 10.1002/art.21269. [DOI] [PubMed] [Google Scholar]
- 23.Ousman SS, et al. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 24.Berger T, et al. Antimyelin antibodies as a predictor of clinically definite multiple sclerosis after a first demyelinating event. N Engl J Med. 2003;349:139–145. doi: 10.1056/NEJMoa022328. [DOI] [PubMed] [Google Scholar]
- 25.Kuhle J, et al. Lack of association between antimyelin antibodies and progression to multiple sclerosis. N Engl J Med. 2007;356:371–378. doi: 10.1056/NEJMoa063602. [DOI] [PubMed] [Google Scholar]
- 26.Lalive PH, et al. Antibodies to native myelin oligodendrocyte glycoprotein are serologic markers of early inflammation in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:2280–2285. doi: 10.1073/pnas.0510672103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Connor KC, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. 2007;12:12. doi: 10.1038/nm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou D, et al. Identification of a pathogenic antibody response to native myelin oligodendrocyte glycoprotein in multiple sclerosis. Proc Natl Acad Sci USA. 2006;103:19057–19062. doi: 10.1073/pnas.0607242103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao YL, Brosnan CF, Raine CS. Experimental autoimmune encephalomyelitis. Qualitative and semiquantitative differences in heat shock protein 60 expression in the central nervous system. J Immunol. 1995;154:3548–3556. [PubMed] [Google Scholar]
- 30.Quintana FJ, Carmi P, Cohen IR. DNA vaccination with heat shock protein 60 inhibits cyclophosphamide-accelerated diabetes. J Immunol. 2002;169:6030–6035. doi: 10.4049/jimmunol.169.10.6030. [DOI] [PubMed] [Google Scholar]
- 31.Quintana FJ, Carmi P, Mor F, Cohen IR. Inhibition of adjuvant arthritis by a DNA vaccine encoding human heat shock protein 60. J Immunol. 2002;169:3422–3428. doi: 10.4049/jimmunol.169.6.3422. [DOI] [PubMed] [Google Scholar]
- 32.van Eden W, van der Zee R, Prakken B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol. 2005;5:318–330. doi: 10.1038/nri1593. [DOI] [PubMed] [Google Scholar]
- 33.Zanin-Zhorov A, et al. Heat shock protein 60 enhances CD4+ CD25+ regulatory T cell function via innate TLR2 signaling. J Clin Invest. 2006;116:2022–2032. doi: 10.1172/JCI28423. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Lucchinetti CF, Bruck W, Lassmann H. Evidence for pathogenic heterogeneity in multiple sclerosis. Ann Neurol. 2004;56:308. doi: 10.1002/ana.20182. [DOI] [PubMed] [Google Scholar]
- 35.Keegan M, et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366:579–582. doi: 10.1016/S0140-6736(05)67102-4. [DOI] [PubMed] [Google Scholar]
- 36.Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: Migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol. 2006;59:880–892. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- 37.Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- 38.Archelos JJ, Hartung HP. Pathogenetic role of autoantibodies in neurological diseases. Trends Neurosci. 2000;23:317–327. doi: 10.1016/s0166-2236(00)01575-7. [DOI] [PubMed] [Google Scholar]
- 39.Wucherpfennig KW, et al. Recognition of the immunodominant myelin basic protein peptide by autoantibodies and HLA-DR2-restricted T cell clones from multiple sclerosis patients. Identity of key contact residues in the B-cell and T-cell epitopes. J Clin Invest. 1997;100:1114–1122. doi: 10.1172/JCI119622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lionel A, et al. Evolution of self-reactive IgG antibody repertoires in patients with relapsing-remitting multiple sclerosis. Immunol Lett. 2005;97:55–62. doi: 10.1016/j.imlet.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 41.Lefranc D, et al. Distortion of the self-reactive IgG antibody repertoire in multiple sclerosis as a new diagnostic tool. J Immunol. 2004;172:669–678. doi: 10.4049/jimmunol.172.1.669. [DOI] [PubMed] [Google Scholar]
- 42.Toyka KV, et al. Myasthenia gravis. Study of humoral immune mechanisms by passive transfer to mice. N Engl J Med. 1977;296:125–131. doi: 10.1056/NEJM197701202960301. [DOI] [PubMed] [Google Scholar]
- 43.Engelhardt B, Ransohoff RM. The ins and outs of T-lymphocyte trafficking to the CNS: Anatomical sites and molecular mechanisms. Trends Immunol. 2005;26:485–495. doi: 10.1016/j.it.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 44.Quintana FJ, et al. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.