Abstract
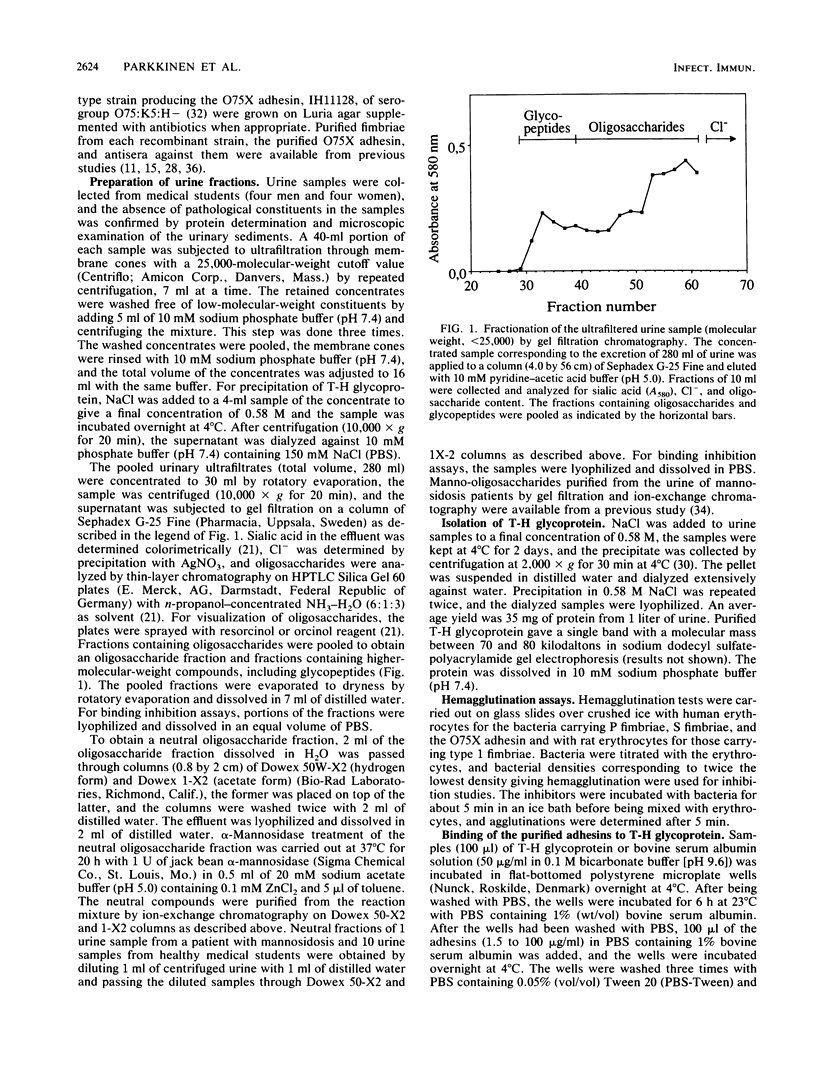
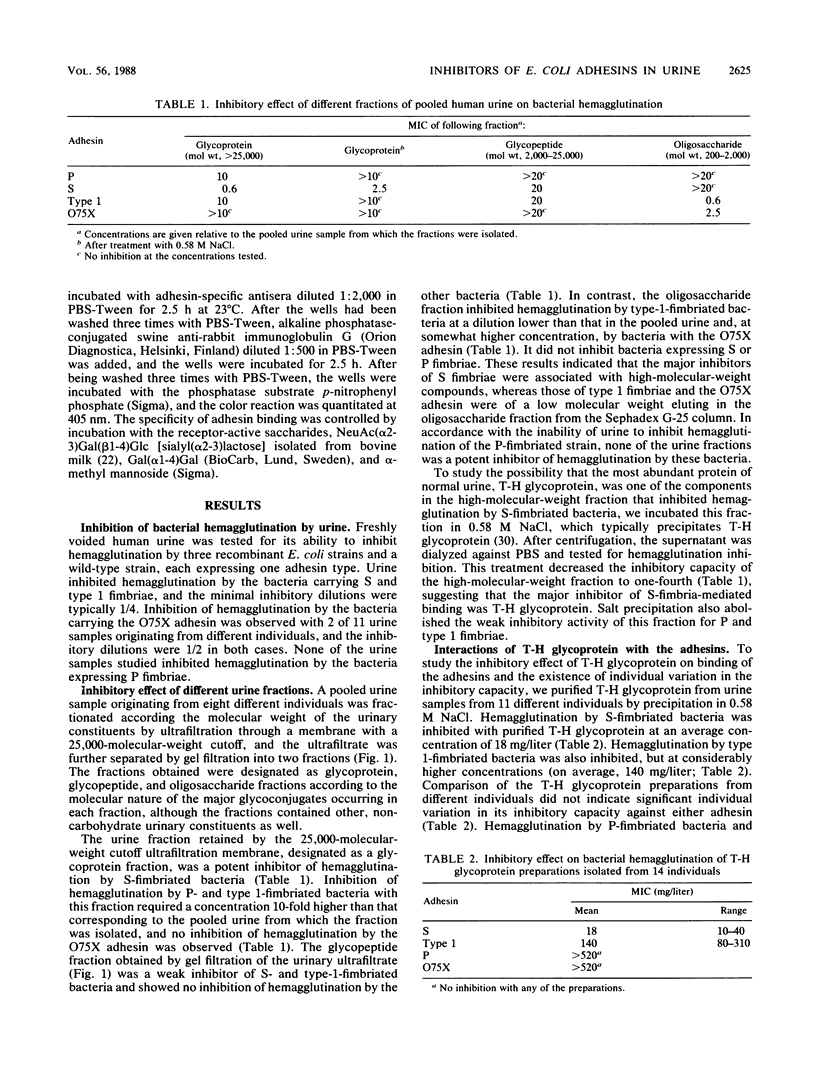
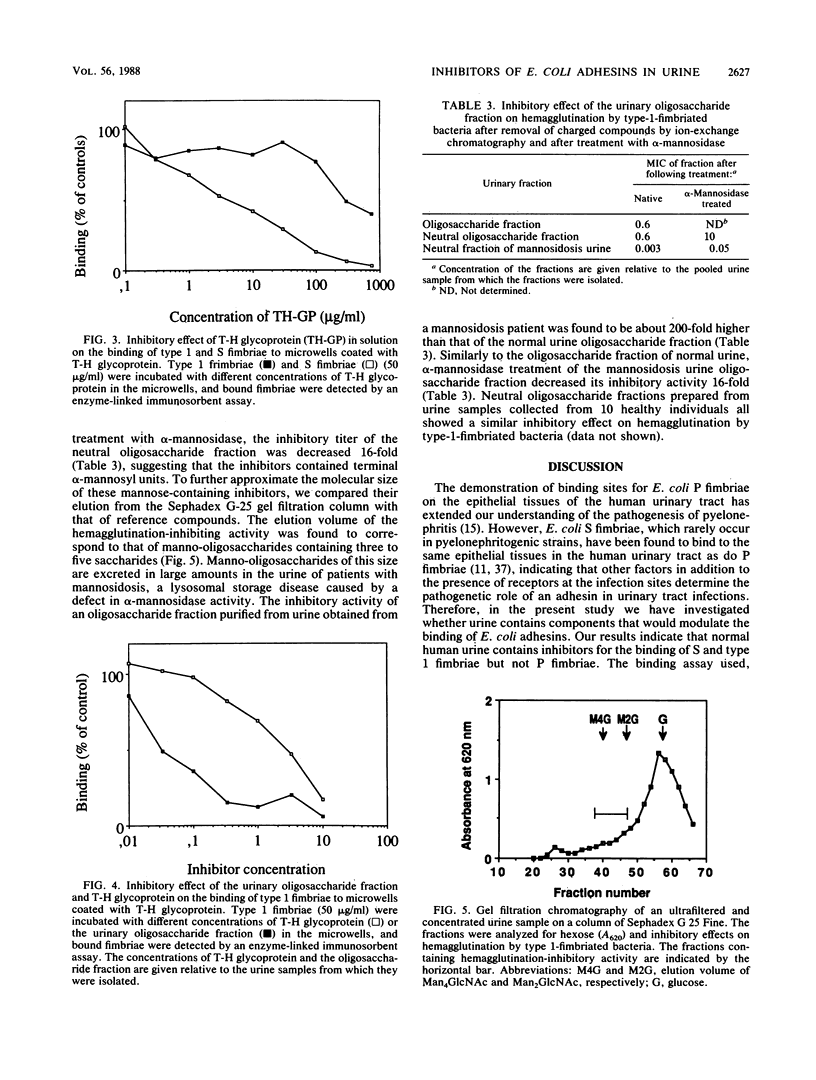
Earlier studies on the binding of Escherichia coli adhesins to the human urinary tract have indicated that the ability to recognize binding sites on the urinary tract epithelial cells is not a characteristic for P fimbriae only, but is also shared by some other adhesins that are not associated with pyelonephritis, especially S fimbriae. In the present study we have investigated whether human urine contains inhibitors of the binding of E. coli adhesins. Normal human urine was found to inhibit hemagglutination by S and type 1 fimbriae but not P fimbriae. The major inhibitor of S fimbriae in normal urine was identified as Tamm-Horsfall glycoprotein, and the interaction with S fimbriae is probably mediated by its sialyloligosaccharide chains. No significant variation was observed in the inhibitory effect of T-H glycoprotein preparations originating from different individuals. In contrast to S fimbriae, the major inhibitors of type 1 fimbriae in urine were identified as low-molecular-weight compounds. Gel filtration and ion-exchange chromatography and alpha-mannosidase treatment indicated that they were neutral alpha-mannosides, probably manno-oligosaccharides with three to five saccharides. Studies of urine samples collected from several individuals indicated the common occurrence of these inhibitory alpha-mannosides. Type 1 fimbriae bound to immobilized T-H glycoprotein, but, unlike S fimbriae, their binding was poorly inhibited by soluble T-H glycoprotein. Some urine samples were also found to contain low-molecular-weight inhibitors for the O75X adhesin of E. coli. These results emphasize that to function as a virulence factor in human urinary tract infections, an adhesin must evidently recognize such receptor structures at the infection sites that are not excreted in soluble form in urine. This prerequisite is filled by P fimbriae but not by type 1 or S fimbriae.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Firon N., Ofek I., Sharon N. Interaction of mannose-containing oligosaccharides with the fimbrial lectin of Escherichia coli. Biochem Biophys Res Commun. 1982 Apr 29;105(4):1426–1432. doi: 10.1016/0006-291x(82)90947-0. [DOI] [PubMed] [Google Scholar]
- Fletcher A. P., Neuberger A., Ratcliffe W. A. Tamm-Horsfall urinary glycoprotein. The chemical composition. Biochem J. 1970 Nov;120(2):417–424. doi: 10.1042/bj1200417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher A. P., Neuberger A., Ratcliffe W. A. Tamm-Horsfall urinary glycoprotein. The subunit structure. Biochem J. 1970 Nov;120(2):425–432. doi: 10.1042/bj1200425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A. M., Neuberger A. The development of a radioimmunoassay for the measurement of urinary Tamm-Horsfall glycoprotein in the presence of sodium dodecyl sulphate. Clin Sci. 1973 Feb;44(2):163–179. doi: 10.1042/cs0440163. [DOI] [PubMed] [Google Scholar]
- Hacker J., Schmidt G., Hughes C., Knapp S., Marget M., Goebel W. Cloning and characterization of genes involved in production of mannose-resistant, neuraminidase-susceptible (X) fimbriae from a uropathogenic O6:K15:H31 Escherichia coli strain. Infect Immun. 1985 Feb;47(2):434–440. doi: 10.1128/iai.47.2.434-440.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Engberg I., Freter R., Lam J., Olling S., Svanborg Edén C. Ascending, unobstructed urinary tract infection in mice caused by pyelonephritogenic Escherichia coli of human origin. Infect Immun. 1983 Apr;40(1):273–283. doi: 10.1128/iai.40.1.273-283.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., Falkow S., Freter R., Svanborg Edén C. Contribution of adhesion to bacterial persistence in the mouse urinary tract. Infect Immun. 1983 Apr;40(1):265–272. doi: 10.1128/iai.40.1.265-272.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. R., Roberts P. L., Stamm W. E. P fimbriae and other virulence factors in Escherichia coli urosepsis: association with patients' characteristics. J Infect Dis. 1987 Jul;156(1):225–229. doi: 10.1093/infdis/156.1.225. [DOI] [PubMed] [Google Scholar]
- Keith B. R., Maurer L., Spears P. A., Orndorff P. E. Receptor-binding function of type 1 pili effects bladder colonization by a clinical isolate of Escherichia coli. Infect Immun. 1986 Sep;53(3):693–696. doi: 10.1128/iai.53.3.693-696.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Parkkinen J., Hacker J., Finne J., Pere A., Rhen M., Holthöfer H. Binding of Escherichia coli S fimbriae to human kidney epithelium. Infect Immun. 1986 Nov;54(2):322–327. doi: 10.1128/iai.54.2.322-327.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Valtonen M. V., Parkkinen J., Väisänen-Rhen V., Finne J., Orskov F., Orskov I., Svenson S. B., Mäkelä P. H. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect Immun. 1985 May;48(2):486–491. doi: 10.1128/iai.48.2.486-491.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Virkola R., Holthöfer H. Localization of binding sites for purified Escherichia coli P fimbriae in the human kidney. Infect Immun. 1986 Nov;54(2):328–332. doi: 10.1128/iai.54.2.328-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen-Rhen V., Rhen M., Pere A., Parkkinen J., Finne J. Escherichia coli fimbriae recognizing sialyl galactosides. J Bacteriol. 1984 Aug;159(2):762–766. doi: 10.1128/jb.159.2.762-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen T. K., Väisänen V., Saxén H., Hultberg H., Svenson S. B. P-antigen-recognizing fimbriae from human uropathogenic Escherichia coli strains. Infect Immun. 1982 Jul;37(1):286–291. doi: 10.1128/iai.37.1.286-291.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Winberg J., Hultberg H. Identification of a carbohydrate receptor recognized by uropathogenic Escherichia coli. Infection. 1980;8 (Suppl 3):288–293. doi: 10.1007/BF01639597. [DOI] [PubMed] [Google Scholar]
- McQueen E. G. The nature of urinary casts. J Clin Pathol. 1962 Jul;15(4):367–373. doi: 10.1136/jcp.15.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hanley P., Lark D., Falkow S., Schoolnik G. Molecular basis of Escherichia coli colonization of the upper urinary tract in BALB/c mice. Gal-Gal pili immunization prevents Escherichia coli pyelonephritis in the BALB/c mouse model of human pyelonephritis. J Clin Invest. 1985 Feb;75(2):347–360. doi: 10.1172/JCI111707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Ferencz A., Orskov F. Tamm-Horsfall protein or uromucoid is the normal urinary slime that traps type 1 fimbriated Escherichia coli. Lancet. 1980 Apr 19;1(8173):887–887. doi: 10.1016/s0140-6736(80)91396-3. [DOI] [PubMed] [Google Scholar]
- Parkkinen J., Finne J., Achtman M., Väisänen V., Korhonen T. K. Escherichia coli strains binding neuraminyl alpha 2-3 galactosides. Biochem Biophys Res Commun. 1983 Mar 16;111(2):456–461. doi: 10.1016/0006-291x(83)90328-5. [DOI] [PubMed] [Google Scholar]
- Parkkinen J., Finne J. Isolation and structural characterization of five major sialyloligosaccharides and a sialylglycopeptide from normal human urine. Eur J Biochem. 1983 Nov 2;136(2):355–361. doi: 10.1111/j.1432-1033.1983.tb07749.x. [DOI] [PubMed] [Google Scholar]
- Parkkinen J., Finne J. Isolation of sialyl oligosaccharides and sialyl oligosaccharide phosphates from bovine colostrum and human urine. Methods Enzymol. 1987;138:289–300. doi: 10.1016/0076-6879(87)38024-3. [DOI] [PubMed] [Google Scholar]
- Parkkinen J., Korhonen T. K., Pere A., Hacker J., Soinila S. Binding sites in the rat brain for Escherichia coli S fimbriae associated with neonatal meningitis. J Clin Invest. 1988 Mar;81(3):860–865. doi: 10.1172/JCI113395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkinen J., Rogers G. N., Korhonen T., Dahr W., Finne J. Identification of the O-linked sialyloligosaccharides of glycophorin A as the erythrocyte receptors for S-fimbriated Escherichia coli. Infect Immun. 1986 Oct;54(1):37–42. doi: 10.1128/iai.54.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pere A., Leinonen M., Väisänen-Rhen V., Rhen M., Korhonen T. K. Occurrence of type-1C fimbriae on Escherichia coli strains isolated from human extraintestinal infections. J Gen Microbiol. 1985 Jul;131(7):1705–1711. doi: 10.1099/00221287-131-7-1705. [DOI] [PubMed] [Google Scholar]
- Pere A., Nowicki B., Saxén H., Siitonen A., Korhonen T. K. Expression of P, type-1, and type-1C fimbriae of Escherichia coli in the urine of patients with acute urinary tract infection. J Infect Dis. 1987 Oct;156(4):567–574. doi: 10.1093/infdis/156.4.567. [DOI] [PubMed] [Google Scholar]
- Sikri K. L., Foster C. L., Bloomfield F. J., Marshall R. D. Localization by immunofluorescence and by light- and electron-microscopic immunoperoxidase techniques of Tamm-Horsfall glycoprotein in adult hamster kidney. Biochem J. 1979 Sep 1;181(3):525–532. doi: 10.1042/bj1810525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAMM I., HORSFALL F. L., Jr A mucoprotein derived from human urine which reacts with influenza, mumps, and Newcastle disease viruses. J Exp Med. 1952 Jan;95(1):71–97. doi: 10.1084/jem.95.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vauhkonen M., Viitala J., Parkkinen J., Rauvala H. High-mannose structure of apolipoprotein-B from low-density lipoproteins of human plasma. Eur J Biochem. 1985 Oct 1;152(1):43–50. doi: 10.1111/j.1432-1033.1985.tb09161.x. [DOI] [PubMed] [Google Scholar]
- Virkola R., Westerlund B., Holthöfer H., Parkkinen J., Kekomäki M., Korhonen T. K. Binding characteristics of Escherichia coli adhesins in human urinary bladder. Infect Immun. 1988 Oct;56(10):2615–2622. doi: 10.1128/iai.56.10.2615-2622.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen-Rhen V., Elo J., Väisänen E., Siitonen A., Orskov I., Orskov F., Svenson S. B., Mäkelä P. H., Korhonen T. K. P-fimbriated clones among uropathogenic Escherichia coli strains. Infect Immun. 1984 Jan;43(1):149–155. doi: 10.1128/iai.43.1.149-155.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Väisänen-Rhen V. Fimbria-like hemagglutinin of Escherichia coli O75 strains. Infect Immun. 1984 Nov;46(2):401–407. doi: 10.1128/iai.46.2.401-407.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Tachibana Y., Mihara K., Okada S., Yabuuchi H., Kobata A. Urinary oligosaccharides of mannosidosis. J Biol Chem. 1980 Jun 10;255(11):5126–5133. [PubMed] [Google Scholar]


