Abstract
Nitric oxide (NO) is involved in number of physiological and pathological events. Our previous studies demonstrated a differential expression of NO signaling components in mouse and human ES cells. Here, we demonstrate the effect of NO donors and soluble guanylyl cyclase (sGC) activators in differentiation of ES cells into myocardial cells. Our results with mouse and human ES cells demonstrate an increase in Nkx2.5 and myosin light chain (MLC2) mRNA expression on exposure of cells to NO donors and a decrease in mRNA expression of both cardiac-specific genes with nonspecific NOS inhibitor and a concomitant increase and decrease in the mRNA levels of sGC α1 subunit. Although sGC activators alone exhibited an increase in mRNA expression of cardiac genes (MLC2 and Nkx2.5), robust inductions of mRNA and protein expression of marker genes were observed when NO donors and sGC activators were combined. Measurement of NO metabolites revealed an increase in the nitrite levels in the conditioned media and cell lysates on exposure of cells to the different concentrations of NO donors. cGMP analysis in undifferentiated stem cells revealed a lack of stimulation with NO donors. Differentiated cells however, acquired the ability to be stimulated by NO donors. Although, 3-(4-amino-5-cyclopropylpyrimidin-2-yl)-1-(2-fluorobenzyl)-1H-pyrazolo [3,4-b]pyridine (BAY 41-2272) alone was able to stimulate cGMP accumulation, the combination of NO donors and BAY 41-2272 stimulated cGMP levels more than either of the agents separately. These studies demonstrate that cGMP-mediated NO signaling plays an important role in the differentiation of ES cells into myocardial cells.
Keywords: cardiomyogenesis, cyclic GMP, soluble guanylyl cyclase, NO donors, sGC activators
Embryonic stem (ES) cells are derived from the inner cell mass of the preimplantation embryo, and because of their self-renewal and pluripotency, they are thought to revolutionize the field of regenerative medicine (1–3). Although transplantation of stem cells into animal models of cardiac injury and Parkinson's disease has revealed some beneficial effects, a better understanding of the role of specific signaling pathways involved in proliferation and differentiation of ES cells is necessary to improve their use in clinical medicine (4–6). Components of a number of signaling pathways such as Wnt/β-catenin (7), phosphotidyle-inosital 3-kinase (8, 9), MAPK (10), and NO (11) have been shown to regulate proliferation and differentiation of stem cells.
NO is a diffusible short-lived free radical and a signaling molecule with a number of important physiological functions such as smooth muscle relaxation, neurotransmission, and inhibition of platelet aggregation and host defense mechanisms (12). It also plays a role in the pathology of several inflammatory diseases and other pathological conditions such as cancer, diabetes, and neurodegenerative diseases (13, 14). NO plays an important role in the control of heart rate, contractibility, coronary perfusion, and cardiac development (15, 16). It is synthesized by enzymes called nitric oxide synthases such as NOS-1 (neuronal NOS), NOS-2 (inducible NOS), and NOS-3 (endothelial NOS) that catalyze the oxidation of l-arginine into l-citruline with the release of NO. NO can also be measured in biological systems as metabolites of NO and as nitrites and nitrates (17). The NO receptor soluble guanylyl cyclase (sGC) is a heme-containing heterodimer with α and β subunits (12). Binding of NO to the heme prosthetic group of sGC catalyzes the conversion of GTP into the second messenger cGMP that can exert many physiological effects, such as mediating vascular smooth muscle tone and motility, phototransduction, and maintenance of fluid and electrolyte homeostasis by interaction of cGMP with downstream effectors such as a family of cGMP-dependent protein kinases, cGMP-dependent phosphodiesterases, and cyclic nucleotide gated channels (11).
Our previous studies with mouse and human ES cells demonstrated a time-dependent increase in mRNA and protein levels of different subunits of sGC (with the exception of β2 mRNA in H-9 cells) during both mouse and human ES cell differentiation into cells of cardiac lineage. Compared with NOS-1, NOS-2, and NOS-3 mRNA and protein levels were also induced during ES cell differentiation (18, 19), thereby demonstrating the involvement of NO signaling components during differentiation of ES cells into cardiac cells. Previous studies by other investigators (20) have shown increased cardiomyogenesis with NO donors and by delivery of the NOS-2 gene in mouse ES cells.
In this study, we demonstrate the role of NO and cGMP in differentiation of human and mouse ES cells by regulating the expression of the NO receptor sGC. Our studies indicate that manipulation of the NO–cGMP pathway will aid in the differentiation of stem cells into myocardial cells.
Results
Effect of NO Donors, NOS Inhibitor, and sGC Activators on Mouse ES Cell Differentiation.
Analyses of markers of differentiation.
To determine the role of the NO signaling pathway in differentiation of ES cells into myocardial cells, we conducted studies with both mouse and human ES cells. Our initial results demonstrate that when mouse ES cells (EZ1) were subjected to differentiation and exposed to slow release NO donors NOC-18 (DETA NONOate; 1 μM) and S-nitroso-N-acetyl-penicillamine (SNAP; 25 μM) on days 0, 5 and 7, and harvested on day 10, there was a 15–19% increase in mRNA expression of cardiac-specific transcription factor Nkx2.5 compared with untreated or DMSO-treated differentiated cells. This result was further supported by Western blot analysis that demonstrated that NOC-18 increased or NG-nitro-l-arginine methyl ester (l-NAME) decreased MHC protein levels (data not shown) during differentiation of EZ1 cells. Similarly, when we examined the effect of the allosteric sGC activator 3-(4-amino-5-cyclopropylpyrimidin-2-yl)-1-(2-fluorobenzyl)-1H-pyrazolo[3,4-b]pyridine] (BAY 41-2272) (1–3 μM; in this case the differentiated cells were harvested on day 8) 65% to 2-fold increases in Nkx2.5 mRNA expression compared with untreated cells were observed. Similarly, in RW-4 mES cells, the NO donor SNAP (25 μM) showed a 1.5-fold increase in Nkx2.5 mRNA expression compared with untreated cells during differentiation. Our results demonstrated no significant change in Nkx2.5 mRNA when cells were exposed to the sGC-specific inhibitor 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ); however, another sGC inhibitor, NS2028, showed a ≈2-fold increase in Nkx2.5 mRNA compared with DMSO-treated cells (data not shown).
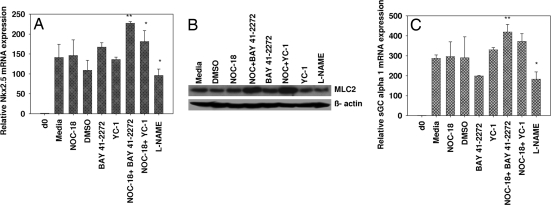
In another independent experiment, EZ-1 cells were treated with either NOC-18 (1 μM), sGC activators BAY 41-2272 or (3-(5′-hydroxymethyl-2′-furyl)-1-benzyl indazole (YC-1; 3 μM), or the combination of NOC-18+BAY 41-2272 or NOC-18+YC-1. The combination of NO donor and sGC activators showed an additive increase in Nkx2.5 mRNA expression (66–108%) compared with DMSO alone. In contrast, NOC-18 alone caused only a slight increase (3.5%), and YC-1 and BAY 41-2272 showed a 25–53% increase in Nkx2.5 mRNA expression compared with cells exposed media or DMSO (Fig. 1A). l-NAME (1 mM), a nonspecific NOS inhibitor, exhibited 32% decrease in Nkx2.5 mRNA compared with the untreated cells. In addition, protein levels of the cardiac marker MLC2 in EZ1 cells exposed to the sGC activators, NO donors, and NOS inhibitor demonstrated a robust increase when cells were exposed to the combination of NOC-18+BAY 41-2272 and NOC-18+YC-1 compared with increases with NOC-18, BAY 41-2272, and YC-1 alone. l-NAME showed a slight inhibition in MLC2 protein levels (Fig. 1B).
Fig. 1.
Effect of an NO donor, sGC activators, and a NOS inhibitor on EZ1 (murine ES) cells. The EZ1 cells were differentiated and exposed to NOC-18 (1 μM), BAY 41-2272, YC-1 (3 μM), l- NAME (1 mM), and a combination of NOC + BAY 41-2272. or NOC + YC-1 on days 0, 5, and 7 and harvested on day 10. (A and C) mRNA levels for the indicated genes were analyzed by real-time PCR and normalized by using the housekeeping gene GAPDH and presented as fold expression compared with day 0. Day 0 represents undifferentiated cell culture usually collected before subjecting cells to differentiation. The data were analyzed by using the 2−ΔΔCt method. Error bars indicate ± SEM. Significance using paired Student's t test is indicated: *, P < 0.05; **, P < 0.01. n = 3. (B) The protein from EZ1 cells untreated or treated with NOC-18 (1 μM), BAY 41-2272, YC-1 (3 μM), l- NAME (1 mM), or the combination exposed on days 0, 5, and 7 and harvested on day 10 was detected with specific antibodies against MLC2 and β-actin by using Western blot–ECL analysis.
Changes in the expression of sGC components.
When differentiated EZ1 cells were evaluated for sGC β1 mRNA expression, 12% to 44% increases in sGC β1 mRNA expression were observed on exposure of cells to SNAP and NOC-18, respectively. However, BAY 41-2272-treated cells exhibited a 11–17% decrease in sGC β1 mRNA expression. In RW-4 mES cells, the NO donor SNAP (25 μM) also showed a 2-fold increase in NOS-2 and a 1.5-fold increase in sGC α1 mRNA expression compared with untreated cells (data not shown).
In another experiment, the combination of NOC-18 (1 μM) and allosteric sGC activators BAY 41-2272 and YC-1 (3 μM) showed an enhanced increase in the mRNA expression of sGC α1 (28–45%) compared with DMSO alone in EZ1 cells. BAY 41-2272 alone (3 μM) showed a 31% decrease, whereas YC-1 showed a 14% increase in sGC α1 mRNA expression compared with cells exposed to vehicle control. In contrast, exposure of cells to l-NAME (1 mM) caused a 36% decrease in sGC α1 mRNA expression (Fig. 1C). These results collectively demonstrate the role of the NO and cGMP signaling components in differentiation of mouse ES cells into myocardial cells.
Effect of NO Donors, NOS Inhibitor, and sGC Activators on Human ES Cell Differentiation.
Analyses of markers of differentiation.
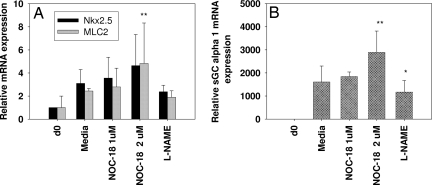
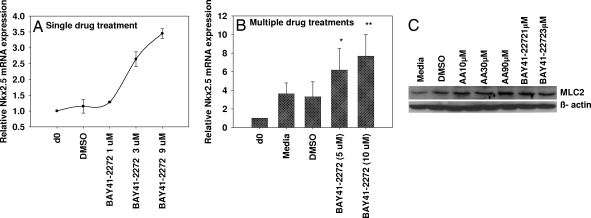
We next evaluated the effect of activators/inhibitors of the NO-cGMP pathway in differentiation of human ES (H-9) cells into myocardial cells. H-9 cells were subjected to differentiation by using embryoid body (EB) formation in ultra-low attachment plate for 5 days, and the EBs were transferred to gelatin-coated plates for further differentiation for an additional 8–14 days. To study the influence of NO donors and inhibitors on the differentiation, we initially exposed H-9 cells to 1–2 μM of the slow release NO donor NOC-18 and the nonspecific NOS-inhibitor l-NAME (2 mM) on day 17 and harvested the cells 24 h later and analyzed the samples by using real-time PCR. Our results (Fig. 2A) indicate a >50% increase and 15–25% decrease in the mRNA expression of cardiac-specific transcription factor Nkx2.5 and MLC2 with NOC-18 and l-NAME, respectively. In addition, exposure of cells to NOC-18 (days 0 and 2) and multiple exposures to SNAP (days 7, 9, 11, and 13) showed 2- to 5-fold increases in the mRNA expression of transcription factor Nkx2.5 and cardiac-specific marker MLC2 (data not shown). To evaluate the role of NO-independent sGC activators (BAY 41-2272 or YC-1) in differentiation, partially differentiated H-9 cells were subjected to either single or multiple treatments with either of the 2 agents. Fig. 3A demonstrates a dose-dependent induction of Nkx2.5 mRNA expression with BAY41-2272 (1–9 μM) when cells were treated for 24 h (day 13) and harvested on day 14. In another independent experiment (Fig. 3B) a 3- to 4-fold increase in mRNA expression of the Nkx2.5 gene was observed when the cells were subjected to multiple treatments of BAY 41-2272 on days 7, 9, 11, and 13. Similarly, another sGC activator, YC-1, showed a 3- to 4-fold increase in Nkx2.5 mRNA compared with untreated control. In addition, we also observed a 2- to 3-fold increase in protein levels of the cardiac marker MLC2 when differentiated cells were exposed to 1–3 μM BAY 41-2272 compared with the untreated controls. Ascorbic acid was used as a positive control that also showed (unpublished observation) an increase in the MLC2 protein levels (Fig. 3C).
Fig. 2.
Effect of NOC-18 and l-NAME in H-9 cells. Human ES cells (H-9) were subjected to differentiation by using the EB method, and the differentiated cells were exposed to an NO donor, NOC-18 (1–2 μM), and a nonspecific NOS inhibitor, l-NAME (2 mM), on day 17 for 24 h. mRNA expression for the indicated genes (MLC2, NkX2.5, sGC α1, and GAPDH) was analyzed by real-time PCR. All of the samples were normalized by using housekeeping gene GAPDH, and data were analyzed by the 2−ΔΔCt method. Error bars (±SEM) indicate significance using paired Student's t test is indicated. *, P < 0.05; **, P < 0.01; n = 4.
Fig. 3.
Effect of BAY 41-2272 on Nkx2.5 mRNA on H-9 cells. (A and B) Partially differentiated H-9 cells were exposed to a single treatment on day 13 (A) or multiple treatments (starting on day 7, total number of exposures 4 (B). The cells were harvested on day 14, and mRNA expression of Nkx2.5 was determined by real-time PCR. All of the samples were normalized with the housekeeping gene GAPDH. *, P < 0.05; **, P < 0.01; n = 3–6. (C) The protein from differentiated H-9 cells either untreated or treated with BAY 41-2272 (1–3 μM) or ascorbic acid (30–90 μM) on days 7, 9, 11, and 13 and harvested on day 14 was extracted and detected with specific antibodies against MLC2 and β-actin by using Western blot–ECL analysis.
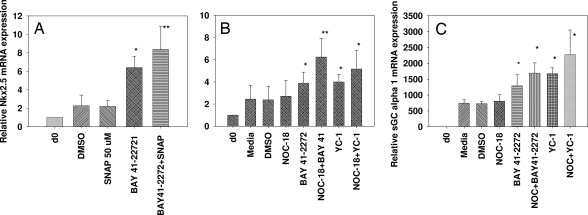
Because NO donors and sGC activators combined have been shown to exhibit synergistic responses in other cell systems, we were interested in evaluating their effects in human ES cell differentiation. To study the combined effect of an NO donor and sGC activator in H-9 differentiation, we exposed the cells to SNAP (50 μM), BAY 41-2272 (10 μM), and the combination of SNAP + BAY 41-2272 on day 13 and harvested the cells on day 14 (24-h treatment) and analyzed the mRNA levels by RT-PCR. Our results demonstrated no significant change in the Nkx2.5 mRNA levels with SNAP alone. BAY 41-2272 showed a >2.5-fold increase, whereas the combination of the 2 showed a marked effect (≈4-fold increase compared with DMSO alone) in modulating Nkx2.5 mRNA expression (Fig. 4A). In addition when partially differentiated cells (treatment started on day 7) were exposed to submaximal concentrations (Fig. 4B) of either NOC-18, sGC activators, or the combination of NOC (1 μM) + BAY 41-2272 (3 μM) and NOC (1 μM) + YC-1(3 μM), the combination of NOC-18 + BAY 41-2272 significantly enhanced the Nkx2.5 mRNA (3.7-fold) expression compared with either of the 2 agents alone (BAY 41-2272 caused a 1.7-fold increase, whereas NOC-18 alone did not show any increase in the Nkx2.5 mRNA relative to untreated cells at 1 μM concentration) compared with DMSO control. In contrast, the combination of YC-1+NOC-18 showed only a modest increase in Nkx2.5 mRNA expression (2.1- to 2.4-fold compared with DMSO alone).
Fig. 4.
Effect of NO donors and sGC activators in H-9 cells. Partially differentiated cells were exposed to a single treatment of the NO donor SNAP (50 μM), BAY 41-2272 (5 μM), and the combination of SNAP+BAY41 (A) or multiple treatments of NOC-18 (1 μM), BAY 41-2272 (3 μM), or the combination of NOC-18+BAY41, YC-1 (3 μM) or the combination of NOC-18 + YC-1 (B and C). The samples were analyzed for Nkx2.5 (A and B) and sGC α1 transcript levels (C) by quantitative RT-PCR. Error bars indicate ± SEM. Significance using paired Student's t test is shown: *, P < 0.05; **, P < 0.01. n = 5–7.
Changes in the expression of sGC components.
Quantitative measurement of NO metabolites in biological samples provides information regarding in vivo NO production. Therefore, we analyzed the production of nitrite levels in conditioned media and cell lysate of differentiated stem cells exposed to NO donors NOC-18 and SNAP for 24 h at 37 °C. Differentiated H-9 cells were exposed to the agents on day 17, and cell lysate and conditioned media were collected on day 18. Our results demonstrate a dose-dependent increase in the nitrite levels in conditioned media on exposure of differentiated cells to NO donors NOC-18 and SNAP (relative fold changes of 15.5–75 with NOC-18, 2–10 μM; 23.5-fold change with SNAP, 25 μM). We also observed a >2-fold increase in the intracellular nitrite levels in differentiated cell lysates with 2–5 μM NOC-18 and a 32% decrease with the nonspecific NOS inhibitor l-NAME as determined by HPLC analysis (data not shown). Moreover, when the samples were analyzed for sGC α1 mRNA expression (NO receptor), there was a 15–80% increase with NOC-18 and 27% decrease with l-NAME compared with the media-alone control (Fig. 2B), demonstrating the role of NO in the differentiation of human ES cells into myocardial cells. In another experiment, compared with DMSO control, SNAP exhibited a 1.4-fold increase in sGC α1 mRNA expression, BAY 41-2272 showed a 1.8-fold increase in sGC α1 mRNA expression, and the combination of BAY 41-2272+SNAP exhibited a 2.37-fold increase in sGC α1 mRNA expression (data not shown).
Similarly, NO donors (2 μM) and sGC activators (3 μM) (submaximal concentrations) and the combination of the 2 showed 77% to 2.5-fold increases in sGC α1 mRNA expression compared with untreated or DMSO-treated control (Fig. 4C) [BAY 41-2272 (77%), YC-1 (1.4-fold), combination of NOC+BAY 41-2272 (>2-fold), and NOC+YC-1 (>2.5-fold]. However, the combination of NO donors and cGMP analogs did not show additive or synergistic effect in mediating differentiation of human ES cells into myocardial cells [supporting information (SI) Fig. S1]. These studies demonstrate that the NO and cGMP pathway may be involved in differentiation of human ES cells into cells of cardiac lineage. However, non-cGMP pathways may also play some role in differentiation. Although there was not a direct correlation with increases in the differentiation and beating myocardial cells, most of these agents, especially NO donors, YC-1 and the combination of NOC + BAY 41-2272, increased the number of beating differentiated cells. Compared with NOC-18 that showed 2 beating areas in cardiac bodies, the combination of NOC + BAY41-2272 showed 7 beating areas in the monolayer of cardiac cells (Movies S1 and S2).
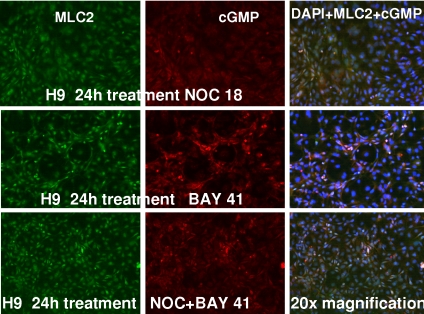
We next conducted immunostaining studies to further confirm the expression of cardiac-specific markers and NO signaling components during H-9 differentiation. Our results (Fig. 5) show the positive staining of MLC2 (cardiac marker) and cGMP in the differentiated cells treated with either NOC-18 (1 μM), BAY 41-2272 (3 μM), or the combination of NOC + BAY 41-2272 for 24 h at 37 °C. Fig. 5 demonstrates cytoplasmic staining with MLC2 and both cytoplasmic and perinuclear staining with cGMP in the NOC-18- and BAY 41-2272-treated group. The combination of NOC-18 + BAY 41-2272 revealed increased numbers of colocalized staining in cells stained with MLC2 and cGMP (in the merged images in Fig. 5) compared with the either of the 2 agents alone (Fig. 5). Control samples with secondary antibody alone (data not shown) did not detect the antigens, and there were no significant differences in the staining of cells exposed to media alone and NOC-18-treated cells. Our results also demonstrate that, in contrast to predominant cytoplasmic sGC α1 staining, modest perinuclear staining of the sGC β1 subunit is also observed in differentiated H-9 cells (Fig. S2). However, the cellular heterogeneity of differentiating cells observed adds complexity that is inherent in these studies.
Fig. 5.
Immunofluorescence detection of MLC2 and cGMP in H-9 cells exposed to NO donor and sGC activator. Differentiated cells were exposed to NOC-18 (1 μM; Top), BAY 41-2272 (3 μM; Middle), or the combination of the two (C) for 24 h at 37 °C. The cells were fixed with paraformaldehyde and incubated with antibodies to MLC2 and cGMP that was followed by detection with fluorescent-conjugated secondary anti-mouse or rabbit antibodies. (Magnification: 20×.)
cGMP Levels in Response to NO Donors and sGC Activator in Undifferentiated and Differentiated Human ES Cells.
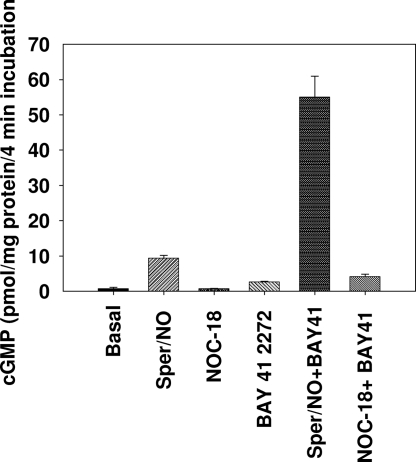
To examine the role of cGMP in differentiation of stem cells into myocardial cells, the basal and stimulated cGMP production was examined in undifferentiated and differentiated H-9 cells. The cells were exposed to NOC-18 (slow release NO donor), NOC-22 (fast-release NO donor), BAY 41-2272, and the combination of the NO donors and BAY 41-2272 for the indicated times. A 10-min pretreatment with 1 mM 3-isobutyl-1-methylxanthine (IBMX) with 0.1% (vol/vol) DMSO or 5 μM BAY 41–2272 was followed by 4-min incubation with 2 μM NOC-18 and 100 μM NOC-22. Fifty microliters of cell suspension was mixed with 50 μL of ice-cold perchloric acid, and cGMP accumulation was measured by ELISA. Our results demonstrate that although undifferentiated cells have high basal cGMP levels, stimulation of undifferentiated cells with NO did not increase cGMP levels any further (5.1 ± 1.4 vs. 4.8 ± 1.3 pmol per mg of protein). We had reported that undifferentiated cells have no detectable sGC (18, 19); therefore, the levels of cGMP in undifferentiated cells are probably caused by particulate guanylyl cyclase. In contrast, although differentiated cells exhibited 7-fold lower levels of basal cGMP compared with undifferentiated cells, a 13-fold stimulation in cGMP accumulation was observed with 100 μM NOC-22, demonstrating that differentiated cells acquire the ability to be stimulated with NO. However, the slow-release NO donor NOC-18 (half-life 24 h in aqueous solutions) was unable to stimulate the cGMP levels as expected. Although, the sGC allosteric activator BAY 41-2272 alone stimulated the cGMP levels by 4-fold, the combination of NOC-22 and BAY 41-2272 showed a robust stimulation (78.5-fold increase compared with basal levels, ≈6-fold increase to NOC-22 alone, 20-fold increase to BAY 41-2272 alone). Similarly, NOC-18 + BAY 41-2272 showed increased stimulation in cGMP levels compared with either of the 2 agents alone. These results clearly demonstrate that a combination of NO donors and BAY 41-2272 markedly increases cGMP levels better than either of the agents alone (Fig. 6).
Fig. 6.
cGMP accumulation in undifferentiated and differentiated H-9 cells. H-9 cells were harvested with 1 mM EDTA in DPBS and incubated for 10 min at 37 °C in DPBS containing 1 mM IBMX with 0.1% DMSO or 5 μM BAY 41-2272 (2–3 × 107 cells per mL) followed by the addition of NO donors. Cells were incubated for 4 min and then 50 μL of ice-cold 1 M perchloric acid was added and cGMP was determined by ELISA. Data are expressed in pmol cGMP per mg of protein.
Discussion
NO–cGMP signaling is a widely studied pathway in many tissues and cells, and reduced production and function of NO has been shown to participate in a number of disorders such as cardiovascular, pulmonary, endothelial, renal, and hepatic diseases and erectile dysfunction. Some of these disorders are treated with “nitrovasodilators” that release NO by spontaneous decomposition or metabolism to activate its receptor sGC (12). Therefore, based on these previous observations, we hypothesized that the NO–cGMP pathway should influence the differentiation of ES cells into cells of various lineages such as myocardial cells, neural precursor cells, and possibly cells of other lineages. Our previous studies clearly indicate the involvement of NO signaling components in the differentiation of both mouse and human ES cells into cardiomyocytes (18, 19).
In the current study, we examined the role of activators and inhibitors of the NO and cGMP pathway in the differentiation of ES cells into myocardial cells, and we have shown that NO donors and sGC activators alone and in combination are able to influence the differentiation of both mouse and human ES cells into myocardial cells better than either of the agents separately. Previous studies have also shown the influence of NO on the differentiation of mouse ES cells into cardiomyocytes by use of NO donors and introduction of the iNOS gene into mouse ES cells (20). NO donors and the compounds that activate sGC in an NO-independent manner might offer considerable advantages to the stem cell field. We have demonstrated the combined effect of NO donors and and sGC activators BAY 41-2272 or YC-1 in the differentiation of human ES cells into myocardial cells. The development of YC-1 and its derivative BAY 41-2272 (21–24), both NO-independent modulators of sGC, have been used as tools to study the function of this enzyme. The function and distribution of sGC has been studied in many systems. Previous studies have shown that sGC expression is present in the vascular endothelial and smooth muscle cells in neonatal heart but expression shifts to endothelial cells in adult heart (24). In addition, although undetectable sGC activity has been shown in rat cardiomyocytes compared with whole heart, activity was stimulated with NO or combination of YC-1/NO (24), demonstrating the integrity of the enzyme. In this study, we have clearly shown that a slow-release NO donor, NOC-18, provided a modest increase in the differentiation of both mouse and human ES cells into myocardial cells. sGC activators BAY 41-2272 or YC-1 caused a 3- to 4-fold increase in the mRNA expression of the cardiac-specific transcription factor Nkx2.5 and cardiac markers MLC2 and MHC in different experiments. However, the combination of NOC-18 + BAY 41-2272 or YC-1 showed an enhanced expression of the maker genes and proteins. In addition, compared with the NO donor NOC-22 and BAY 41-2272, a robust stimulation of cGMP accumulation was observed when the 2 agents were used in combination. In addition, although the slow-release NO donor NOC-18 alone did not show stimulation of cGMP levels, when it was combined with BAY 41-2272 there was a 4-fold increase in cGMP accumulation compared with BAY 41-2272 alone, demonstrating the presence of an activatable sGC as has been described (24) in isolated rat cardiomyocytes. BAY 41-2272 by itself shows some increase in cGMP levels, possibly because of increased NOS-2 and NOS-3 levels in differentiated cells (18, 19).
Some studies have addressed the importance of NO–cGMP signaling in development. Previous investigation by Bloch et al. (16) suggested the importance of NOS-2-derived NO production in the cardiomyocyte differentiation of mouse ES cells. Similarly, introduction of the NOS- 2 gene via adenoviral vector in mouse ES cells has been shown to facilitate cardiomyocyte production (25). In addition, recently the NO–cGMP pathway has been implicated in the differentiation of stem cells into cells of various lineages in response to various plant compounds. Zhu and Lou (25) demonstrated that icariin (a constituent of Epimedium, a traditional Chinese medicine) induced the differentiation of mouse ES cells into cardiomyocytes by the elevation of the cAMP/cGMP ratio in ES cells and up-regulation of the endogenous generation of NO during the early stages of cardiac development. Similarly, the plant compound genistein has been shown to stimulate osteoblastic differentiation in bone marrow culture via the NO–cGMP pathway (26).
Based on our previous studies with mouse and human ES cells (18, 19), in addition to the preliminary studies of others with mouse ES cells, we believe that the NO–cGMP signaling system has a significant role in the differentiation of mouse and human stem cells into cells of various lineages including cardiomyocytes. Although the inherent heterogeneity of cell population in differentiating stem cells obviously complicates the correlation of biochemistry with cell biology with our studies and others, important clues are forthcoming that may permit us and others to pharmacologically influence stem cell differentiation, tissue engineering, and transplantation biology.
Materials and Methods
Antibodies.
sGC α1, and anti-β-actin were purchased from Sigma, and anti-MLC2 and MHC were purchased from Santa Cruz Biotechnology.
Cell Culture and Differentiation of Mouse ES Cells.
EZ-1 mouse ES cells derived from the 129 S1/Sv ImJ strain (gift from Eva Zsigmond, Institute of Molecular Medicine, Houston) were maintained on mitotically inactivated murine embryonic fibroblast (MEF) and cultured in DMEM supplemented with nonessential amino acids, Hepes buffer, sodium pyruvate, l-glutamine, 15% defined FBS (HyClone), 1/75,000 dilution of monothioglycerol (Sigma) solution, and 1,000 units/mL of leukemia inhibitory factor (Chemicon). The cells were passaged on MEF 2–3 times before transferring them onto gelatin-coated plates. A modified method of Hescheler et al. (3) was used for the EB formation and differentiation. EZ1 cells were treated with DMSO, NOC-18 (1–2 μM, NO donor), l-NAME (1–2 mM, nonspecific NOS-inhibitor), BAY 41-2272 YC-1 (3 μM, sGC activators), 8-bromo-cGMP (100 μM), ODQ, and NS2028 (10–20 μM sGC inhibitors) on day 0 (undifferentiated), day 5, and day 7 (contracting regions within EBs were identified by day 6 onward). Finally, the differentiated cells were harvested on day 10 and analyzed by using real-time PCR and Western blot analysis.
Cell Culture and Differentiation of Human ES Cells.
H-9 (WA-09, human ES) were purchased from WiCell Research Institute and grown in 80% DMEM/F12, 20% knockout serum replacer, 1 mM l-glutamine, 0.1 mM β-mercaptoethanol, and 1 mM nonessential amino acids supplemented with 4 ng/ml bFGF on mitotically-inactivated MEF feeder layers and matrigel. For cardiomyocyte differentiation, the cells were dissociated by using 2 mg/ml of collagenase IV (Invitrogen), washed, and cultured in suspension in low attachment plates (Corning) in the differentiation medium (80% K/O-DMEM, 1 mM l-glutamine, 0.1 mM ß- mercaptoethanol, 1 mM nonessential amino acids, and 20% defined FBS) (HyClone). The media were changed on days 2 and 4, and on day 6 the EBs were transferred onto gelatin-coated plates (3–4 EBs /cm2) and cultured for additional days as described in Results, and day 0 was designated as the undifferentiated ES cells. Human ES cell-derived cardiomyocytes and other heterogeneous populations of the cells were analyzed by immunostaining, RT-PCR, and Western blot analyses.
Treatment of Partially Differentiated Cells with the Activators and Inhibitors of the NO Pathway.
To determine whether the activators and inhibitors of the NO pathway would influence the differentiation of H-9 cells into myocardial cells, EBs and partially differentiated cells were incubated with various concentrations of NO donors NOC-18 (1–2 μM), NOC-22 (100 μM), SNAP (25–50 μM), nonspecific NOS inhibitor l-NAME (2 mM), or allosteric sGC activators BAY 41-2272 (3–10 μM) and YC-1 (3–10 μM) on either days 0 or 2 (early), days 7, 9, 11, or 13 (multiple treatments), or days 13–17 (single treatment).
Real-Time RT-PCR.
Total RNA from undifferentiated and differentiated cells was isolated by using ultraSpec total RNA isolation reagent (Biotecx). cDNA was prepared by using a high-capacity cDNA archive kit (Applied Biosystems), according to the manufacturer's suggestions. Real-time RT-PCR assays for different subunits of sGC (α1, β1) Nkx2.5, MLC2, and GAPDH were purchased from Applied Biosystems and determined by using the manufacturer's suggested protocol. All reactions were conducted with the 7900 HT Prizm Sequence Detection System for 40 cycles. The results were analyzed using the 2−ΔΔCt method (27).
Western Blot Analysis.
Cell cultures were washed with cold PBS and collected in cell extraction buffer (Invitrogen) supplemented with 1 mM PMSF and protease inhibitor mixture (Sigma) for 30 min on ice. Equal protein aliquots were resolved on SDS/PAGE and transferred onto nitrocellulose membrane. The membranes were blocked for 45 min in a blocking buffer (5% milk in Tris-buffered saline), washed, and incubated with specific antibodies either overnight at 4 °C (most) or for 30–60 min (β-actin) at room temperature. Proteins were detected with HRP-conjugated secondary antibodies and visualized by enhanced chemiluminescence.
Immunostaining.
H-9 cells grown in gelatin-coated chamber slides (partially differentiated cells), exposed to NOC-18 (2 μM), BAY 41-2272 (3 μM), or a combination of the two for 24 h at 37 °C were quickly washed with ice-cold PBS and fixed with 2% paraformaldehyde as described (18, 19) Staining of primary antibodies was detected by using Alexa-fluor fluorescent-labeled secondary antibodies (Molecular Probes) and inverted fluorescent microscopy. Digital images were captured with a Zeiss fluorescence microscope and Zeiss imaging software.
cGMP Assay in Human ES Cells (H-9).
Cells were washed with Dulbecco's PBS (DBPS) and harvested in DPBS containing 1 mM EDTA. Cell pellet was resuspended in DPBS containing 1 mM IBMX with 0.1% (vol/vol) DMSO or 5 μM BAY41-2272 (2–3 × 107 cells per ml) and incubated for 10 min at 37 °C. Then, NOC-22 or NOC18 were added to the cell suspensions to make final concentrations of 100 and 2 μM, respectively. Cells were incubated for 4 min, and 50 μL of the cell suspension was mixed with 50 μL of ice-cold 1 M perchloric acid. cGMP was extracted on ice for 30 min. Supernatant fractions were neutralized with potassium carbonate (12.5 μL of 2 M) and used for cGMP assay by ELISA as described (28). Pellets were dissolved in 0.5 mL of 0.1 M NaOH and used to measure protein by the BioRad dye-binding assay. Data were expressed in pmol cGMP per mg of protein.
Nitrite concentrations were quantified by ion chromatography (ENO20 Analyzer; Eicom) (17).
Supplementary Material
Acknowledgments.
This work was supported in part by National Institutes of Health Grant R01 GM076695 (to F.M.), the John S. Dunn Foundation, and the University of Texas.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810230105/DCSupplemental.
References
- 1.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1997;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 2.Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: Somatic differentiation in vitro. Nat Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- 3.Hescheler J, et al. Embryonic stem cells: A model to study structural and functional properties in cardiomyogenesis. Cardiovasc Res. 1997;36:149–162. doi: 10.1016/s0008-6363(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 4.Min JY, et al. Transplantation of embryonic stem cells improves cardiac function in postinfarcted rats. J Appl Physiol. 2002;92:288–296. doi: 10.1152/jappl.2002.92.1.288. [DOI] [PubMed] [Google Scholar]
- 5.Min JY, et al. Long-term improvement of cardiac function in rats after infarction by transplantation of embryonic stem cells. J Thorac Cardiovasc Surg. 2003;125:361–369. doi: 10.1067/mtc.2003.101. [DOI] [PubMed] [Google Scholar]
- 6.Kim JH, et al. Dopaminergic neurons derived from embryonic stem cells function in an animal model of Parkinson's disease. Nature. 2002;418:50–56. doi: 10.1038/nature00900. [DOI] [PubMed] [Google Scholar]
- 7.Singh AM, et al. Chibby, an antagonist of the Wnt/β-catenin pathway, facilitates cardiomyocyte differentiation of murine embryonic stem cells. Circulation. 2007;15:617–626. doi: 10.1161/CIRCULATIONAHA.106.642298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sauer H, Rahimi G, Hescheler J, Wartenberg M. Role of reactive oxygen species and phosphatidylinositol 3-kinase in cardiomyocyte differentiation of embryonic stem cells. FEBS Lett. 2000;76:218–233. doi: 10.1016/s0014-5793(00)01747-6. [DOI] [PubMed] [Google Scholar]
- 9.Klinz F, Bloch W, Addicks K, Hescheler J. Inhibition of phosphatidyl-inositol 3-kinase blocks development of functional embryonic cardiomyocytes. Exp Cell Res. 1999;274:79–83. doi: 10.1006/excr.1998.4309. [DOI] [PubMed] [Google Scholar]
- 10.Li J, et al. MEK/ERK signaling contributes to the maintenance of human embryonic stem cell self-renewal. Differentiation. 2007;75:299–307. doi: 10.1111/j.1432-0436.2006.00143.x. [DOI] [PubMed] [Google Scholar]
- 11.Krumenacker JS, Murad FM. NO-cGMP signaling in development and stem cells. Mol Genet Metab. 2006;87:311–314. doi: 10.1016/j.ymgme.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Murad F. Shattuck Lecture: Nitric oxide and cyclic GMP in cell signaling and drug development. N Engl J Med. 2006;355:2003–2011. doi: 10.1056/NEJMsa063904. [DOI] [PubMed] [Google Scholar]
- 13.Davis KL, Martin E, Turko IV, Murad F. Novel effects of nitric oxide. Annu Rev Pharmacol Toxicol. 2001;41:203–236. doi: 10.1146/annurev.pharmtox.41.1.203. [DOI] [PubMed] [Google Scholar]
- 14.Krumenacker JS, Hanafy KA, Murad F. Regulation of nitric oxide and soluble guanylyl cyclase. Brain Res Bull. 2004;62:505–515. doi: 10.1016/S0361-9230(03)00102-3. [DOI] [PubMed] [Google Scholar]
- 15.Kojda G, et al. Protein expression, vascular reactivity and soluble guanylate cyclase activity in mice lacking the endothelial cell nitric oxide synthase: Contributions of NOS isoforms to blood pressure and heart rate control. Cardiovasc Res. 1999;42:206–213. doi: 10.1016/s0008-6363(98)00315-0. [DOI] [PubMed] [Google Scholar]
- 16.Bloch W, et al. Nitric oxide synthase expression and role during cardiomyogenesis. Cardiovasc Res. 1999;43:675–684. doi: 10.1016/s0008-6363(99)00160-1. [DOI] [PubMed] [Google Scholar]
- 17.Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radical Biol Med. 2007;43:645–657. doi: 10.1016/j.freeradbiomed.2007.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krumenacker JS, Katsuki S, Kots A, Murad F. Differential expression of genes involved in cGMP-dependent nitric oxide signaling in murine embryonic stem (ES) cells and cell-derived cardiomyocytes. Nitric Oxide. 2006;14:1–11. doi: 10.1016/j.niox.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Mujoo K, Krumenacker JS, Wada Y, Murad F. Differential expression of nitric oxide signaling components in undifferentiated and differentiated human embryonic stem cells. Stem Cells Dev. 2006;15:779–787. doi: 10.1089/scd.2006.15.779. [DOI] [PubMed] [Google Scholar]
- 20.Kanno S, et al. Nitric oxide facilitates cardiomyogenesis in mouse embryonic stem cells. Proc Natl Acad Sci USA. 2004;101:12277–12281. doi: 10.1073/pnas.0401557101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM. YC-1, a novel activator of platelet guanylate cyclase. Blood. 1994;84:4226–4233. [PubMed] [Google Scholar]
- 22.Stasch JP, et al. NO-independent regulatory site on soluble guanylate cyclase. Nature. 2001;410:212–215. doi: 10.1038/35065611. [DOI] [PubMed] [Google Scholar]
- 23.Evgenov OV, et al. NO- independent stimulators and activators of soluble guanylate cyclase: Discovery and therapeutic potential. Nat Rev Drug Discov. 2006;5:755–768. doi: 10.1038/nrd2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behrends S, et al. The expression pattern of nitric oxide-sensitive guanylyl cyclase in rat heart changes during postnatal development. J Histochem Cytochem. 2002;50:1325–1331. doi: 10.1177/002215540205001005. [DOI] [PubMed] [Google Scholar]
- 25.Zhu DY, Lou YJ. Icariin-mediated expression of cardiac genes and modulation of nitric oxide signaling pathway during differentiation of mouse embryonic stem cells into cardiomyocytes in vitro. Acta Pharmacol Sin/I. 2006;27:311–320. doi: 10.1111/j.1745-7254.2006.00275.x. [DOI] [PubMed] [Google Scholar]
- 26.Pan W, et al. Genistein stimulates the osteoblastic differentiation via NO/cGMP in bone marrow culture. J Cell Biochem. 2005;94:307–316. doi: 10.1002/jcb.20308. [DOI] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Kots, et al. Pyridopyrimidine derivatives as inhibitors of cyclic nucleotide synthesis: Application for treatment of diarrhea. Proc Natl Acad Sci USA. 2008;105:8440–8445. doi: 10.1073/pnas.0803096105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.