Abstract
Pancreatic ductal adenocarcinoma (PDAC) is believed to arise through a multistep model comprised of putative precursor lesions known as pancreatic intraepithelial neoplasia (PanIN). Recent genetically engineered mouse models of PDAC demonstrate a comparable morphologic spectrum of murine PanIN (mPanIN) lesions. The histogenesis of PanIN and PDAC in both mice and men remains controversial. The most faithful genetic models activate an oncogenic KrasG12D knockin allele within the pdx1- or ptf1a/p48-expression domain of the entire pancreatic anlage during development, thus obscuring the putative cell(s)-of-origin from which subsequent mPanIN lesions arise. In our study, activation of this knockin KrasG12D allele in the Elastase- and Mist1-expressing mature acinar compartment of adult mice resulted in the spontaneous induction of mPanIN lesions of all histological grades, although invasive carcinomas per se were not seen. We observed no requirement for concomitant chronic exocrine injury in the induction of mPanIN lesions from the mature acinar cell compartment. The acinar cell derivation of the mPanINs was established through lineage tracing in reporter mice, and by microdissection of lesional tissue demonstrating Cre-mediated recombination events. In contrast to the uniformly penetrant mPanIN phenotype observed following developmental activation of KrasG12D in the Pdx1-expressing progenitor cells, the Pdx1-expressing population in the mature pancreas (predominantly islet β cells) appears to be relatively resistant to the effects of oncogenic Kras. We conclude that in the appropriate genetic context, the differentiated acinar cell compartment in adult mice retains its susceptibility for spontaneous transformation into mPanIN lesions, a finding with potential relevance vis-à-vis the origins of PDAC.
Keywords: lineage tracing, transdifferentiation, precursor lesions, pancreatic cancer
The basis for the moniker “pancreatic ductal adenocarcinoma” (PDAC) is morphologic, and is attributable to misplaced attempts by the neoplastic cells to recapitulate normal tubulo-ductal structures in the pancreas. Multiple lines of evidence suggest that PDAC does not arise de novo, but rather progresses through a multistep model comprised of noninvasive precursor lesions known as pancreatic intraepithelial neoplasia (PanIN), culminating in invasive cancer (1). Despite the remarkable advances made in understanding the molecular pathogenesis of pancreatic cancer and that of its putative precursor lesions, the histogenesis of PDAC has remained a contentious issue for many years (2, 3). Not surprisingly, some of the early literature on this topic implied a cell-of-origin within the ductal epithelium (4), as reiterated by the dominant morphologic features and the expression of various immunophenotypic markers of ductal differentiation within these cells, and the compelling epidemiological association with intraductal precursor lesions (5). Nevertheless, early experiments targeting an oncogenic Kras allele to cytokeratin 19 (CK19)-expressing pancreatic ductal epithelium of mice failed to yield a neoplastic phenotype, with the caveat that the mutant allele was not expressed under endogenous regulatory elements (6). Other studies, based on chemical carcinogenesis models in Syrian hamsters or in rats, have postulated nonductal origins of PDAC, namely from the endocrine and acinar compartments, respectively (7, 8). Because carcinogens can adversely impact multiple cell types in the pancreas, the lineage fidelity from cell-of-origin to eventual neoplasia is hard to preserve (9). Although enforced expression of oncogenes under Elastase regulatory elements in the acinar compartments results in neoplasms with a mixed acinar-ductal histology (10), the nonphysiologic levels of transgene expression, the use of heterologous promoter elements, and the absence of appropriate precursor lesions recapitulating the cognate human disease renders extrapolation of these findings to the origins of human PDAC speculative at best.
A major breakthrough toward understanding the initiation and progression of PDAC was enabled by recent genetically engineered mouse models, wherein a mutant KrasG12D (or KrasG12V) allele is conditionally activated by Cre-mediated recombination within the entire pancreatic anlage during development (11, 12). Because the knockin oncogene is expressed from its endogenous regulatory elements, it mimics physiologic expression levels observed in human disease. These mice develop the histological compendium of putative precursor lesions, which are designated as murine PanIN or “mPanIN” lesions, to distinguish them from their human counterparts (13). Multiple compound transgenic models generated subsequently have established a requirement for mutant Kras to develop PDACs in the backdrop of mPanIN lesions, with the additional genetic “hit” typically accelerating the progression to invasive cancer and lethality (14–16). While isolated misexpression of certain oncogenes (for example, Gli2) (17) or loss of tumor suppressor genes (for example, Pten) (18) does result in pancreatic neoplasms with variable differentiation, classical mPanINs are not found in these pancreata, underscoring a possibly unique ability of mutant Kras to facilitate transformation of a susceptible pancreatic cell type(s) into the mPanIN phenotype.
In the aforementioned reports (14–16), mutant Kras is activated during development in either the Pdx1 or the ptf1/p48 expression domains, by means of Cre-mediated recombination. Pdx1 and ptf1/p48 are transcription factors with “pan”-epithelial expression in the pancreas during morphogenesis (19), and therefore, progenitor cells within all three epithelial compartments (acini, ducts, and islets) are liable to undergo recombination. Owing to the nature of the recombination event, mutant Kras will continue to be expressed in all surviving progeny, even if Pdx1 (or ptf1/p48) expression per se is extinguished in these cells. As a result, it has been technically challenging to identify the precise cell type(s) that might be susceptible to mPanIN formation in the postnatal period. In addition, because adult pancreata are the biologically relevant soil for most sporadic PDAC in humans, and genetic events predisposing to this malignancy are highly unlikely to occur during development, it is desirable to recapitulate this scenario in the context of murine pancreatic neoplasia. In a recent study, Guerra and colleagues targeted an oncogenic KrasG12V allele to the Elastase-expression domain of either developing or adult mouse pancreata, both leading to the formation of mPanIN lesions and metastatic adenocarcinoma (20). Surprisingly, while developmental activation resulted in spontaneous generation of mPanINs and cancers, the adult pancreas was refractory to oncogenic Kras expressed from its endogenous promoter elements, unless further challenged by chronic exocrine injury induced by cerulein. Owing to the limitations of lineage tracing, the investigators were unable to further delineate between the two Elastase-expressing populations—acinar cells versus centroacinar cells (CACs) (21)—as the cell-of-origin for the observed lesions. Nevertheless, this report establishes that the Elastase-expressing nonductal compartment of the mature pancreas is capable of generating bone fide mPanIN lesions, although the combination of genetic (mutant Kras) and environmental (chronic pancreatitis) factors appeared to be an absolute prerequisite for ductal neoplasia in this model.
We conducted this study with several objectives: first, to better delineate the nonductal population in the mature pancreas that yields a “ductal” preneoplastic phenotype (i.e., mPanINs) upon mutant KrasG12D expression; second, to investigate the requirement for concomitant exocrine injury in inducing such preneoplastic lesions; and finally, to determine whether the activation of mutant KrasG12D in the adult Pdx1 expression domain (predominantly islet β-cells) recapitulates the phenotype observed with developmental expression. We used two independent tamoxifen-inducible Cre mice that restricted KrasG12D activation to the mature acinar compartment. Similarly, tamoxifen-inducible Cre mice were used for KrasG12D activation in the Pdx1-expressing cells within the adult pancreas. We observed the spontaneous development of mPanIN lesions of all histological grades in the pancreata of adult mice with acinar-restricted KrasG12D expression, whereas no lesions were observed with KrasG12D expressed in the Pdx1 population. Notably, mPanIN lesions were induced in the absence of concurrent exocrine injury, underscoring the spontaneous ability of mature acinar cells to transition to a ductal precursor phenotype in the appropriate oncogenic context. Our findings have considerable significance for understanding the cellular histogenesis of PDAC.
Results
Spontaneous mPanIN Formation upon Acinar Cell-Specific Activation of Mutant Kras from Its Endogenous Promoter Elements.
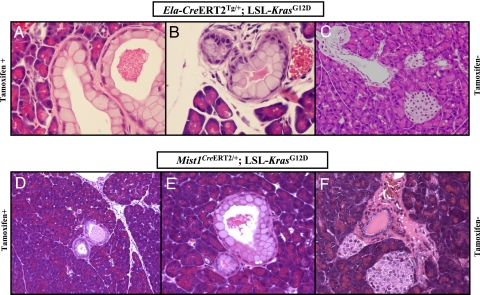
To induce Cre-mediated recombination, cohorts of bitransgenic mice were induced at 6 weeks of age with tamoxifen. Two noninduced Ela-CreERT2Tg/+; LSL- KrasG12D and Mist1CreERT2/+; LSL- KrasG12D littermates were also killed at each of the six time points (from 2 months to 12 months post-induction), and these did not display any pancreatic phenotype (Fig. 1 C and F, respectively). In contrast, following tamoxifen-induction in the Ela-CreERT2Tg/+; LSL- KrasG12D cohort, focal low grade mPanIN lesions were observed in 3 of 5 (60%) mice as early as 2 months after injection of tamoxifen (Fig. 1 A and B). The mPanIN lesions had no nuclear atypia and demonstrated abundant cytoplasmic mucin without papillary projections, consistent with grade mPanIN-1A, as previously described (13). Nearly indistinguishable low-grade mPanIN lesions were observed in the pancreata of Mist1CreERT2/+; LSL-KrasG12D mice at a comparable age of 2 months post-induction (Fig. 1 D and E), underscoring a commonality of histogenesis in both genetic models. Of note, the mPanIN lesions in either background occurred in the absence of any histological evidence of ongoing pancreatic injury (i.e., no acute or chronic pancreatitis was seen). With advancing age (4 to 12 months post-induction), acinar expression of mutant Kras resulted in uniformly penetrant mPanIN lesions of all histological grades, including mPanIN-2 (Fig. 2 A and B) and mPanIN-3 (“carcinoma-in situ”) (Fig. 2 C and D). Previously described features of higher grade mPanIN lesions included loss of nuclear polarity, presence of mitoses, cribriforming, and micropapillary structures lined by highly atypical ductal epithelium (13). The mPanIN lesions observed in the later time points often occurred in the backdrop of lobulocentric atrophy, comparable to what has been described in human PanIN lesions (22). No invasive neoplasms were observed in either cohort of tamoxifen-induced mice at the culmination of this study.
Fig. 1.
Targeting KrasG12D to mature acinar cells results in spontaneous development of murine PanIN (mPanIN) lesions. (A) Low-grade mPanIN lesion (mPanIN-1A) in an Ela-CreERT2Tg/+; LSL-KrasG12D mouse, at 2 months posttamoxifen induction. (B) A second example of a low-grade mPanIN lesion (mPanIN-1A) in an Ela-CreERT2Tg/+; LSL-KrasG12D mouse. Features of low-grade mPanIN lesions illustrated in A and B includex the basally located nuclei, retained nuclear polarity, and absence of nuclear pleomorphism or mitoses. In addition, the abundant intracellular mucin is a characteristic feature. Note the absence of either lobular atrophy or histological evidence of pancreatitis in the surrounding parenchyma. (C) Absence of a discernible pancreatic phenotype in a noninduced Ela-CreERT2Tg/+; LSL-KrasG12D mouse, at 12 months of age. (D) Low-grade mPanIN lesion (mPanIN-1A) in a Mist1CreERT2/+; LSL-KrasG12D mouse, at 2 months posttamoxifen induction. The overall histological features are almost identical to those observed in the Ela-CreERT2Tg/+; LSL-KrasG12D mice, including the absence of either lobular atrophy or histological evidence of pancreatitis in the surrounding parenchyma. (E) A second example of a low-grade mPanIN lesion (mPanIN-1A) in a Mist1CreERT2/+; LSL-KrasG12D mouse, at 2 months post-tamoxifen induction. The diagnostic features of low-grade mPanIN are present. (F) Absence of a discernible pancreatic phenotype in a noninduced Mist1CreERT2/+; LSL- KrasG12D mouse, at 12 months of age.
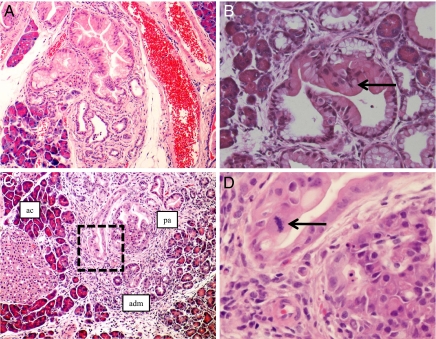
Fig. 2.
The entire histological spectrum of mPanINs is observed with acinar targeting of mutant KrasG12D. (A) An example of high-grade mPanIN lesion (mPanIN-2) in the pancreas of an Ela-CreERT2Tg/+; LSL-KrasG12D mouse, harvested at 12 months posttamoxifen induction. The mPanIN-2 arises on the backdrop of lobulocentric trophy, while normal acinar parenchyma is seen toward the periphery. (B) An example of high-grade mPanIN lesion (mPanIN-2) in the pancreas of a Mist1CreERT2/+; LSL-KrasG12D mouse, harvested at 12 months post-tamoxifen induction. Micropapillary architecture with loss nuclear polarity is discernible (arrow). (C) An example of the highest grade mPanIN lesion (mPanIN-3, or carcinoma-in situ) in an Ela-CreERT2Tg/+; LSL-KrasG12D mouse, at 12 months posttamoxifen induction. Areas of histologically normal acinar parenchyma (ac) merge into acinar ductal metaplasia (adm), which in turn, transition into the high-grade mPanIN lesion (pa). (D) High-power view of the boxed area within the mPanIN-3 lesion illustrated in B, demonstrating the presence of a mitotic figure (arrow), nuclear pleomorphism, and loss of nuclear polarity.
Acinar-Ductal Metaplasia and Biphenotypic Differentiation Are Observed in Acinar Cell-Derived mPanIN Lesions.
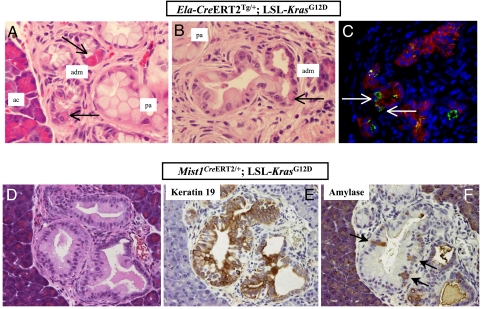
A recent morphological analysis of pancreata in 4-week-old ptf1aCre/+; LSL-KrasG12D mice had suggested that the acinar compartment might be the proximate source of mPanIN lesions (23). Specifically, this study documented extensive acinar-ductal metaplasia preceding the onset of mPanIN lesions, with the individual metaplastic structures comprised of differentiated acinar cells admixed with mucinous ductal epithelium reminiscent of that seen in low-grade mPanINs. Areas of transition from normal acini to metaplastic epithelium to frank mPanINs were observed in the ptf1aCre/+; LSL-KrasG12D mice, suggestive of a histological progression emanating within the acinar compartment, in the setting of mutant Kras expression. Moreover, individual “biphenotypic” cells expressing markers of acinar and ductal differentiation were observed within the metaplastic ducts, as well as in the more obvious mPanIN lesions, further reiterating a putative acinar derivation. Many of these previously described features in the ptf1aCre/+; LSL-KrasG12D mice were recapitulated upon acinar cell specific expression of mutant Kras in adult pancreata. We found areas of acinar-ductal metaplasia located within the immediate vicinity of mPanIN lesions, with progressive transition from normal acinar parenchyma to metaplastic structures, to mPanINs (Figs. 2C and 3 A and B). These metaplastic structures demonstrated a “hybrid” phenotype of differentiated acinar cells containing prominent zymogen granules intermixed with mucinous cells resembling those observed in the adjacent mPanINs (Fig. 3 A and B). Immunofluorescence studies confirmed the presence of scattered amylase expressing acinar cells within the metaplastic epithelium (Fig. 3C). In low-grade mPanIN lesions, immunohistochemical studies demonstrated that whereas the majority of cells expressed the ductal cytokeratin CK19, occasional single cells coexpressing amylase were also present, confirming biphenotypic exocrine differentiation (Fig. 3 D–F), akin to what has been described in the ptf1aCre/+; LSL-KrasG12D mice (23).
Fig. 3.
Acinar-ductal metaplasia and biphenotypic differentiation are observed in acinar cell-derived mPanIN lesions. (A) Histological sections of pancreata from an Ela-CreERT2Tg/+; LSL-KrasG12D mouse at 6 months post-tamoxifen induction demonstrate acinar-ductal metaplastic lesions (adm) bridging the parenchyma between normal acinar structures (ac) and fully developed mPanINs (pa). The metaplastic structures contain differentiated acinar cells, as evidenced by their coarse zymogen granules (arrows), intermixed with mucinous epithelium characteristic of low-grade mPanIN lesions. (B) Another representative example of acinar ductal metaplasia (adm) in an Ela-CreERT2Tg/+; LSL-KrasG12D mouse at 10 months post-tamoxifen induction, transitioning into a more obvious mPanIN lesion (pa) towards the left of the field. Single acinar cells (arrows) are seen within the metaplastic epithelium. (C) Immunofluorescence with anti-amylase antibody (green channel) confirms the presence of residual acinar cells within metaplastic epithelium (anti-Ecad = red channel). DAPI (blue channel) is used as nuclear counterstain. Pancreatic section from an Ela-CreERT2Tg/+; LSL-KrasG12D mouse at 6 months post-tamoxifen induction. (D) A fully developed mPanIN-2 lesion in a Mist1CreERT2/+; LSL-KrasG12D mouse, at 4 months of age. (E) Immunophenotypic analysis of the mPanIN lesion illustrated in D shows extensive labeling with cytokeratin CK19, a marker of ductal differentiation. (F) Immunophenotypic analysis of the mPanIN lesion illustrated in D shows scattered labeling with amylase (arrows), consistent with biphenotypic acinar-ductal differentiation.
Aberrant Notch Activation Is an Early Molecular Alteration in the Pathogenesis of Acinar Cell-Derived mPanIN Lesions.
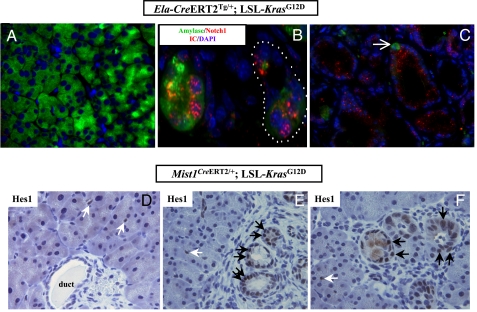
Activation of the Notch signaling pathway is one of the earliest discernible molecular alterations in both human and murine PanIN lesions (11, 24). For example, Notch activation has been reported in the incipient acinar-ductal metaplastic structures before the onset of mPanIN lesions in 4-week-old ptf1aCre/+; LSL-KrasG12D mice (23). Notch misexpression represses terminal acinar cell differentiation in the developing pancreas (25), and mediates acinar-ductal metaplasia in the setting of ectopic growth factor stimulation in the mature pancreas (24). In light of this prior evidence, we assessed Notch activation in metaplastic and mPanIN lesions by using two independent strategies: first, by the expression of cleaved intracytoplasmic domain of Notch 1 receptor (N1-ICD), and second, by expression of the Notch target and basic helix–loop–helix (bHLH) transcription factor, Hes1 (24). In the canonical pathway, ligand binding leads to enzymatic cleavage of the intramembranous portion of the Notch receptor, and nuclear translocation of the ICD, where it binds to and activates a multicomponent transcriptional activator complex. Expression of cytoplasmic or nuclear N1-ICD was essentially absent in the uninvolved parenchyma of tamoxifen-induced Ela-CreERT2Tg/+; LSL- KrasG12D mice (Fig. 4A). In contrast, marked up-regulation of N1-ICD expression was observed in acinar-ductal metaplasia (Fig. 4B), with the most prominent nuclear localization observed in the transitioning amylase-expressing acinar cells immediately bordering the metaplastic, amylase-negative ductal epithelium. Sustained up-regulation of N1-ICD was also seen in fully developed mPanIN lesions, albeit the localization was predominantly cytoplasmic in these structures (Fig. 4C), possibly reflecting a reduced requirement for an active pathway subsequent to completion of the metaplastic event(s). Mirroring the N1-ICD activation in the early metaplastic lesions of Ela-CreERT2Tg/+; LSL- KrasG12D mice, we observed prominent nuclear accumulation of Hes1 in regions of acinar-ductal metaplasia and incipient mPanINs in the Mist1CreERT2/+; LSL-KrasG12D mice (Fig. 4 E and F); in contrast, uninvolved acinar parenchyma did not express Hes1, and labeling in these areas was restricted to the CACs, as expected (Fig. 4D).
Fig. 4.
Activation of the Notch signaling pathway is an early molecular alteration in acinar-derived murine PanIN lesions. (A) No discernible expression of the Notch1 intracytoplasmic domain (N1-ICD, red channel) is seen in the uninvolved acinar parenchyma distant from mPanIN lesions, in a 4-month-old Ela-CreERT2Tg/+; LSL-KrasG12D mouse. DAPI is used as nuclear counterstain (blue channel). (B) Strong nuclear and cytoplasmic expression of N1-ICD is seen in acinar ductal metaplasia from a 4-month-old Ela-CreERT2Tg/+; LSL-KrasG12D mouse. Intense nuclear N1-ICD is particularly prominent in the individual remnant acinar cells, as discernible by their persistent amylase expression, within the metaplastic structures (dotted line). (C) Diffuse cytoplasmic N1-ICD expression in fully formed mPanIN lesions in Ela-CreERT2Tg/+; LSL-KrasG12D mice. Single amylase-expressing acinar cells are evident within the mPanIN lesions (see also Fig. 3). (D) No nuclear Hes1 expression is seen in the uninvolved acinar parenchyma of a 2month old Mist1CreERT2/+; LSL-KrasG12D mouse and the only cells expressing the protein are centroacinar cells (white arrows). The ductal epithelium in the photomicrograph is also Hes1-negative. (E) Uniformly strong nuclear Hes1 expression is observed in acinar ductal metaplasia and incipient mPanIN lesions in a Mist1CreERT2/+; LSL-KrasG12D mouse (black arrows). In contrast, the adjacent uninvolved acinar parenchyma is negative sans the expected labeling in centroacinar cells (white arrows). (F) Additional examples of nuclear Hes1 labeling in acinar ductal metaplasia and incipient mPanIN lesions Mist1CreERT2/+; LSL-KrasG12D mice (black arrows), reiterating the absence of expression in adjacent uninvolved acinar cells.
Discussion
The concept of pancreatic acinar cells as a post-differentiated “dead end” cell type has undergone considerable reassessment in recent years. In particular, studies in experimental models of pancreatitis have confirmed the ability of mature acinar cells to function as so-called facultative progenitors (26, 27). In these injury models, residual acinar cells contribute to the regeneration of the exocrine compartment through an intermediate process of “dedifferentiation”, exemplified by the transient acquisition of a primitive transcriptional program, followed subsequently by “re-differentiation” into mature acinar cells (26). In contrast, rigorous in vivo lineage tracing studies have established that transdifferentiation of acinar cells into nouveau β-cells or ductal epithelium is, for the most part, absent (26, 27). This mirrors the inability of regenerating β-cells to undergo transdifferentiation into exocrine tissues (28, 29), reinforcing the existence of a “like begets like” plasticity in the adult pancreas. One notable exception to these observations occurs in the setting of chronic cerulein-mediated injury, wherein a minor subpopulation of metaplastic ductal epithelium is, in fact, derived via transdifferentiation of acinar cells (27). The acinar-derived metaplastic ducts, termed as “mucinous metaplastic lesions” in the aforementioned study, morphologically resemble low-grade mPanINs (13). Thus, whereas the inherent plasticity of mature acinar cells is mostly lineage-restricted, there is an underlying potential for these cells to differentiate along a true ductal phenotype when placed within the appropriate genetic or environmental context.
In this study, we demonstrate that acinar expression of a mutant KrasG12D allele from its endogenous promoter results in the spontaneous induction of mPanIN lesions in adult mice, sans the requirement for concomitant exocrine injury. Restricted expression of mutant Kras was enabled by using two independent Cre“driver” lines under Elastase and Mist1 regulatory elements, with comparable results across the two genotypes. Multiple previous reports support the ability of acinar cells to undergo neoplastic transformation upon misexpression of mutant Kras under acinar regulatory elements (10, 30). For example, Konieczny and colleagues have described a mouse model of widely metastatic exocrine pancreatic carcinoma of mixed acinar-ductal differentiation in mice harboring a Mist1KrasG12D/+ knockin allele (30). These acinar cell-targeted models, however, do not rely on the endogenous regulatory elements for Kras expression, a critical distinction from the current study. As previously documented, the expression of oncogenic Kras from its endogenous promoter appears to be a prerequisite for developing mPanIN lesions that recapitulate the multistep histological progression of the cognate human disease (11, 12). Akin to mice expressing mutant Kras during pancreatic development, we have shown that differentiated acinar cells in adult mice are capable of generating the entire histological spectrum of mPanIN lesions, including high-grade mPanIN-3 (carcinoma-in situ), without the requirement for exocrine injury. Although invasive neoplasia is not observed in the relatively small cohort of mice aged to 12 months post-tamoxifen induction, these results are not inconsistent with those observed in prior “single-hit” genetically engineered models. For example, in Pdx1-Cre; LSL-KrasG12D mice, only 7% of mice developed PDAC with latency over 1 year, although 100% of the animals developed mPanIN lesions by a few months of age (11). In contrast, cooperating genetic lesions (mutant Ink4/Arf, p53, etc) dramatically alter the natural history of disease in compound transgenic animals, with accelerated and fully penetrant progression to invasive neoplasia (14, 15). Given the presence of mPanIN-3 lesions with KrasG12D expression alone, we believe that the addition of cooperating mutations will similarly enable the development of PDAC in the acinar cell-targeted models.
Over and above the histological resemblance of acinar cell-derived mPanINs to those arising in ptf1aCre/+; LSL-KrasG12D and Pdx1-Cre; LSL-KrasG12D mice, we found additional similarities that suggest a common histogenesis in the developmental and adult preneoplasia models. Thus, “hybrid” metaplastic lesions, containing acinar cells intermixed with mucinous mPanIN-like epithelium, were observed adjacent to mPanIN lesions in both settings, as were the presence of occasional cells with biphenotypic differentiation within fully developed mPanINs (23). Zhu et al. had raised the possibility of acinar cell derivation of mPanIN lesions in their extensive morphological and immunophenotypic analyses of young (4-week-old) ptf1aCre/+; LSL-KrasG12D mice (23); however, the static nature of observations and the “global” recombination within the pancreas precluded definitive assessment of the origin of mPanINs in that report. Lineage tracing studies with Rosa26R reporter mice, as well as molecular demonstration of Cre-mediated recombination in lesional tissue, confirmed that the mPanIN lesions in the current study were, in fact, derivatives of differentiated acinar cells (see Supporting Information). Aberrant activation of the Notch signaling pathway is observed as an early molecular alteration in both human and mouse PanIN lesions (11, 23, 24). Evidence supporting Notch activation is also observed in the current study, with striking nuclear accumulation of N1-ICD and Hes1in the early acinar-ductal metaplastic lesions arising in both models. The Notch pathway has previously been implicated in acinar-ductal transdifferentiation in response to aberrant signaling through the epidermal growth factor receptor (EGFR) pathway (24), and therefore, Notch activation within transdifferentiating acinar cells is not entirely unexpected in the setting of mutant KrasG12D expression. Weijzen et al. have reported that oncogenic Ras up-regulates intracellular N1-ICD, and that the latter is a critical downstream effector of Ras signaling in transformed cells (31). If Notch activation is an absolute requirement for the transdifferentiation of acinar cells expressing mutant Kras into metaplastic ductal epithelium, the forerunners of mPanIN lesions, then this pathway could emerge as an important target for the prevention of PDAC at its earliest stages. Genetic or pharmacological knockdown of Notch signaling should enable addressing this question in an in vivo context.
How do we reconcile the discordance between the absolute requirement for concomitant injury for mPanIN formation within the adult pancreas, as reported by Guerra et al. (20), and the spontaneous generation of mPanINs observed with two independent models in the current study? Briefly, Guerra et al. used a “tet-off” system for conditional induction of a mutant KrasG12V allele from its endogenous promoter in the postnatal exocrine pancreas of Ela-tTA/TetO-Cre; LSL-KrasG12Vgeo mice. The targeting vector used contains an internal ribosomal entry site β-galactosidase (IRES β-geo) cassette inserted into the 3′ untranslated region of the mutant allele, thus permitting bicistronic expression of β-galactosidase from the endogenous Kras promoter (12). In contrast to uniformly penetrant mPanIN lesions observed with developmental KrasG12V expression, young mice maintained on doxycycline till postnatal day 10 developed mPanINs with reduced penetrance and significantly longer latencies, while adult mice maintained on doxycycline till 2 months of age were completely refractory to mPanIN formation, unless exposed to concomitant pancreatic injury. There are several potential explanations for the observed discrepancy in terms of spontaneous mPanIN formation between Ela-tTA/TetO-Cre; LSL-KrasG12Vgeo mice and Ela-CreERT2Tg/+ (or Mist1CreERT2/+); LSL- KrasG12D mice. For example, comparisons between various Ras codon 12 mutations have identified differences in the transforming properties of the G12V versus the G12D alleles (32). Specifically, the valine residue has been shown to induce a fully transformed morphology with more focus formation in Rat-1 rodent cells, compared with the aspartate mutant. However, the enhanced in vitro transforming ability of the G12V allele might come with a price in vivo, in the form of enhanced oncogene-induced senescence (33). Widespread induction of senescence has, in fact, been documented in preneoplastic lesions, including mPanINs, arising in the setting of mutant KrasG12V expression, and markers of senescence are lost only upon progression to invasive carcinomas (34). Although senescence per se has not been systematically evaluated in the KrasG12D models, the highly proliferative nature of early metaplastic lesions in the pancreas (23) suggests that senescence might be bypassed more efficiently, and this could translate into the spontaneous induction of mPanINs in adult mice, without the requirement for exocrine injury. Other potential mechanisms for the observed discrepancy in phenotype could be stochastic interference from the knockin IRES β-geo cassette in the KrasG12V targeting vector, or differences in recombination efficiency between the various Cre driver lines. We emphasize the speculative nature of these explanations. We do not believe that the timing of mutant Kras activation in the adult pancreas is likely to have had significant impact, as they were comparable in both studies (postnatal ages of 2 months vs. 6 weeks, respectively). Irrespective of the requirement for inflammation in Kras-induced tumorigenesis, it is highly likely that concomitant inflammation hastens the acquisition of a neoplastic phenotype, likely through activation of oncogenic signaling pathways like Notch, Hedgehog, and nuclear factor κ B (NFκB), among others (26, 35).
Finally, we wish to emphasize that the observations in the current study do not negate the possibility that mPanINs might also arise after activation of oncogenic Kras in additional nonacinar cell types. For example, Korc and colleagues have demonstrated that activation of oncogenic Kras in nestin-expressing cells also results in mPanIN formation (36). Nestin is a marker of progenitor cells, and nestin-positive progenitors contribute to the formation of the differentiated acinar cells (37). Further, nestin is reexpressed in acinar cells upon “de-differentiation” during epithelial injury and regeneration (26). As a result, activation of oncogenic Kras in the nestin lineage might represent an indirect means of targeting oncogenic Kras to mature acinar cells.
In summary, we have demonstrated that acinar expression of a mutant KrasG12D allele from its endogenous promoter results in the spontaneous induction of the entire histological spectrum of mPanIN lesions in adult mice. Activation of the Notch signaling pathway is one of the earliest demonstrable molecular alterations in acinar cell-derived mPanINs, and the availability of systemic gamma secretase inhibitors that block Notch signaling (38) provide an opportunity for exploring this oncogenic pathway as a preclinical target for PDAC chemoprevention. The extrapolation of our findings to the cognate human disease should not only provide biological insights into the cell-of-origin for PDAC, but also new avenues for therapy and prevention of this lethal malignancy.
Materials and Methods
Genetically Engineered Mice.
All vertebrate animal experiments were conducted in compliance with the National Institutes of Health guidelines for animal research, and approved by the Institutional Animal Care and Use Committees of the Johns Hopkins University School of Medicine and Purdue University. Three tamoxifen-inducible Cre driver strains were used in our study for conditional activation of mutant KrasG12D in the mature pancreas: Ela-CreERT2Tg/+, Pdx1-CreERT2Tg/+, and Mist1CreERT2/+. The Ela-CreERT2Tg/+ and Pdx1-CreERT2Tg/+ strains harbor transgenic (Tg) CreERT2 alleles, while the Mist1CreERT2/+ mice contain a CreERT2 knockin into one Mist1 allele. The three inducible Cre lines were crossed into conditionally activated mutant KrasG12D mice, wherein the oncogene is expressed from its endogenous promoter, as previously described (11). Tamoxifen-mediated CreERT2 induction was performed at 6 weeks of age in cohorts of bitransgenic Ela-CreERT2Tg/+; LSL- KrasG12D, Mist1CreERT2/+; LSL- KrasG12D, and Pdx1-CreERT2Tg/+; LSL- KrasG12D, respectively, by daily intraperitoneal injection of tamoxifen (2 mg/day, Sigma) over five days. Groups of five induced mice from each of the three cohorts were harvested at two-monthly intervals, beginning at 2 months post-tamoxifen injection, and culminating at 12 months. Two noninduced littermates were used as control animal at each time point, for each cohort. A cohort of Mist1CreERT2/+; LSL-KrasG12D; Rosa26R reporter mice were generated for lineage tracing in the spontaneously arising mPanIN lesions.
Note.
We apologize to numerous colleagues whose deserving work could not be referenced in this manuscript due to paucity of space.
Supplementary Material
Acknowledgments.
We thank Dan DiRenzo for use of the CAGpr-LSL-Mist1Myc mice. This work was supported by National Institutes of Health Grants CA113669 and DK072532 (to A.M.), DK61215 and DK56211 (to S.D.L.), and DK55489 and CA124586 (to S.F.K.); the Sol Goldman Pancreatic Cancer Research Center (A.M.); Lustgarten Foundation for Pancreatic Cancer Research (A.M. and S.F.K.); the Phi Beta Psi Sorority Cancer Fund (S.F.K.); a fellowship grant within the Postdoctoral Program of the German Academic Exchange Service (to G.F.); and Ruth L. Kirschstein National Research Service Award T32DK007713 (to R.A.M.). S.D.L. is supported by the Paul Neumann Professorship in Pancreatic Cancer Research.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810097105/DCSupplemental.
References
- 1.Hruban RH, et al. Pancreatic intraepithelial neoplasia: A new nomenclature and classification system for pancreatic duct lesions. Am J Surg Pathol. 2001;25:579–586. doi: 10.1097/00000478-200105000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Longnecker DS, Memoli V, Pettengill OS. Recent results in animal models of pancreatic carcinoma: Histogenesis of tumors. Yale J Biol Med. 1992;65:457–465. [PMC free article] [PubMed] [Google Scholar]
- 3.Pour PM, Pandey KK, Batra SK. What is the origin of pancreatic adenocarcinoma? Mol Cancer. 2003;2:13. doi: 10.1186/1476-4598-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamieson JD. Prospectives for cell and organ culture systems in the study of pancreatic carcinoma. J Surg Oncol. 1975;7:139–141. doi: 10.1002/jso.2930070209. [DOI] [PubMed] [Google Scholar]
- 5.Cubilla AL, Fitzgerald PJ. Morphological lesions associated with human primary invasive nonendocrine pancreas cancer. Cancer Res. 1976;36:2690–2698. [PubMed] [Google Scholar]
- 6.Brembeck FH, et al. The mutant K-ras oncogene causes pancreatic periductal lymphocytic infiltration and gastric mucous neck cell hyperplasia in transgenic mice. Cancer Res. 2003;63:2005–2009. [PubMed] [Google Scholar]
- 7.Pour PM, Schmied B. The link between exocrine pancreatic cancer and the endocrine pancreas. Int J Pancreatol. 1999;25:77–87. doi: 10.1385/IJGC:25:2:77. [DOI] [PubMed] [Google Scholar]
- 8.Bockman DE, et al. Origin and development of the precursor lesions in experimental pancreatic cancer in rats. Lab Invest. 2003;83:853–859. doi: 10.1097/01.lab.0000074918.31303.5a. [DOI] [PubMed] [Google Scholar]
- 9.Murtaugh LC, Leach SD. A case of mistaken identity? Nonductal origins of pancreatic “ductal” cancers. Cancer Cell. 2007;11:211–213. doi: 10.1016/j.ccr.2007.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Grippo PJ, Nowlin PS, Demeure MJ, Longnecker DS, Sandgren EP. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer research. 2003;63:2016–2019. [PubMed] [Google Scholar]
- 11.Hingorani SR, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 12.Guerra C, et al. Tumor induction by an endogenous K-ras oncogene is highly dependent on cellular context. Cancer Cell. 2003;4:111–120. doi: 10.1016/s1535-6108(03)00191-0. [DOI] [PubMed] [Google Scholar]
- 13.Hruban RH, et al. Pathology of genetically engineered mouse models of pancreatic exocrine cancer: Consensus report and recommendations. Cancer Res. 2006;66:95–106. doi: 10.1158/0008-5472.CAN-05-2168. [DOI] [PubMed] [Google Scholar]
- 14.Aguirre AJ, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hingorani SR, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Izeradjene K, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11:229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 17.Pasca di Magliano M, et al. Hedgehog/Ras interactions regulate early stages of pancreatic cancer. Genes Dev. 2006;20:3161–3173. doi: 10.1101/gad.1470806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stanger BZ, et al. Pten constrains centroacinar cell expansion and malignant transformation in the pancreas. Cancer Cell. 2005;8:185–195. doi: 10.1016/j.ccr.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 19.Murtaugh LC, Melton DA. Genes, signals, and lineages in pancreas development. Annu Rev Cell Dev Biol. 2003;19:71–89. doi: 10.1146/annurev.cellbio.19.111301.144752. [DOI] [PubMed] [Google Scholar]
- 20.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 21.Stanger BZ, Dor Y. Dissecting the cellular origins of pancreatic cancer. Cell cycle. 2006;5:43–46. doi: 10.4161/cc.5.1.2291. [DOI] [PubMed] [Google Scholar]
- 22.Brune K, et al. Multifocal Neoplastic Precursor Lesions Associated With Lobular Atrophy of the Pancreas in Patients Having a Strong Family History of Pancreatic Cancer. Am J Surg Pathol. 2006;30:1067–1076. Translated from Eng. [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu L, Shi G, Schmidt CM, Hruban RH, Konieczny SF. Acinar cells contribute to the molecular heterogeneity of pancreatic intraepithelial neoplasia. Am J Pathol. 2007;171:263–273. doi: 10.2353/ajpath.2007.061176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miyamoto Y, et al. Notch mediates TGF alpha-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell. 2003;3:565–576. doi: 10.1016/s1535-6108(03)00140-5. [DOI] [PubMed] [Google Scholar]
- 25.Esni F, et al. Notch inhibits Ptf1 function and acinar cell differentiation in developing mouse and zebrafish pancreas. Development. 2004;131:4213–4224. doi: 10.1242/dev.01280. [DOI] [PubMed] [Google Scholar]
- 26.Fendrich V, et al. Hedgehog signaling is required for effective regeneration of exocrine pancreas. Gastroenterology. 2008;135:347–351. doi: 10.1053/j.gastro.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strobel O, et al. In vivo lineage tracing defines the role of acinar-to-ductal transdifferentiation in inflammatory ductal metaplasia. Gastroenterology. 2007;133:1999–2009. doi: 10.1053/j.gastro.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strobel O, et al. Beta cell transdifferentiation does not contribute to preneoplastic/metaplastic ductal lesions of the pancreas by genetic lineage tracing in vivo. Proc Natl Acad Sci USA. 2007;104:4419–4424. doi: 10.1073/pnas.0605248104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, et al. Beta cells can be generated from endogenous progenitors in injured adult mouse pancreas. Cell. 2008;132:197–207. doi: 10.1016/j.cell.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Tuveson DA, et al. Mist1-KrasG12D knock-in mice develop mixed differentiation metastatic exocrine pancreatic carcinoma and hepatocellular carcinoma. Cancer Res. 2006;66:242–247. doi: 10.1158/0008-5472.CAN-05-2305. [DOI] [PubMed] [Google Scholar]
- 31.Weijzen S, et al. Activation of Notch-1 signaling maintains the neoplastic phenotype in human Ras-transformed cells. Nat Med. 2002;8:979–986. doi: 10.1038/nm754. [DOI] [PubMed] [Google Scholar]
- 32.Seeburg PH, Colby WW, Capon DJ, Goeddel DV, Levinson AD. Biological properties of human c-Ha-ras1 genes mutated at codon 12. Nature. 1984;312:71–75. doi: 10.1038/312071a0. [DOI] [PubMed] [Google Scholar]
- 33.Bardeesy N, Sharpless NE. RAS unplugged: Negative feedback and oncogene-induced senescence. Cancer Cell. 2006;10:451–453. doi: 10.1016/j.ccr.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 34.Collado M, et al. Tumour biology: Senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- 35.Jensen JN, et al. Recapitulation of elements of embryonic development in adult mouse pancreatic regeneration. Gastroenterology. 2005;128:728–741. doi: 10.1053/j.gastro.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 36.Carriere C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci USA. 2007;104:4437–4442. doi: 10.1073/pnas.0701117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Leach SD. Epithelial differentiation in pancreatic development and neoplasia: New niches for nestin and Notch. J Clin Gastroenterol. 2005;39:S78–82. doi: 10.1097/01.mcg.0000155547.83901.a3. [DOI] [PubMed] [Google Scholar]
- 38.van Es JH, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.