Abstract
Lateral roots are initiated postembryonically in response to environmental cues, enabling plants to explore efficiently their underground environment. However, the mechanisms by which the environment determines the position of lateral root formation are unknown. In this study, we demonstrate that in Arabidopsis thaliana lateral root initiation can be induced mechanically by either gravitropic curvature or by the transient bending of a root by hand. The plant hormone auxin accumulates at the site of lateral root induction before a primordium starts to form. Here we describe a subcellular relocalization of PIN1, an auxin transport protein, in a single protoxylem cell in response to gravitropic curvature. This relocalization precedes auxin-dependent gene transcription at the site of a new primordium. Auxin-dependent nuclear signaling is necessary for lateral root formation; arf7/19 double knock-out mutants normally form no lateral roots but do so upon bending when the root tip is removed. Signaling through arf7/19 can therefore be bypassed by root bending. These data support a model in which a root-tip-derived signal acts on downstream signaling molecules that specify lateral root identity.
Keywords: Auxin, auxin transport, mechanosensory, organogenesis, plant gravitropism
Plants are immobile, with the vast majority relying on the mechanical support an extensive root system provides. Lateral roots, formed by postembryonic initiation and development, grow in response to specific environmental cues. In this way, a plant can optimize nutrient and water uptake, whilst avoiding physical obstacles. Thus, root architecture, an important agronomic trait, is to a large extent determined by the environment.
Lateral root initiation (LRI) is dependent on the coordinated action of many genes. Although more than seventy genes of Arabidopsis thaliana have been shown to affect lateral root development, auxin and its polar transport have emerged as central regulators (1, 2). For example, specific Aux/IAA transcriptional regulators appear necessary for lateral root growth. The gain-of-function mutation solitary root (slr-1) encodes a stabilized form of one such Aux/IAA protein, IAA14, which binds and constitutively represses two auxin response factors (ARF7 and ARF19), transcriptional activators also required for lateral root development (3). Consequently, slr-1 plants form no lateral roots. A third gene family, named LATERAL ORGAN BOUNDARIES-DOMAIN (LBD), has also been implicated in auxin-dependent LRI, with LBDs 16 and 29 acting as downstream effectors of ARF7 and ARF19 (4). Although some very early events in lateral root development such as cell division, redirection of auxin flux and cell differentiation are well documented (1, 2), the factors that determine where a new root will be initiated are unknown. Recent work has revealed a genetic basis for the initiation of lateral roots. Root waving, LRI, and a temporal pattern of auxin maxima (as measured by the auxin-sensitive DR5 promoter) are correlated in the root tip, resulting in lateral roots being spaced along the main axis in a regular left-right alternating pattern. Under standardized experimental conditions which negate any extrinsic environmental effect on LRI, it has been proposed that fluctuations in auxin distribution might mediate the lateral roots' regular longitudinal spacing (5).
How are these cues integrated to shape the root system in response to the environment? The following experiments demonstrate that LRI is a consequence of root bending, either in response to gravity or by direct manipulation. Here, we report on the series of molecular events that follow lateral root induction, with respect to auxin transport, auxin signaling, and the acquisition of stem cell identity within the new lateral root primordium.
Results and Discussion
Lateral Root Positioning is Consistent with Root Shape.
To investigate the basis for the interaction between root architecture and the environment, we first examined the relationship between mechanical strain and LRI. It is known that in A. thaliana, when grown on hard agar plates tilted at 45°, lateral root establishment coincides with a waving pattern in the growing root, with lateral roots always emerging on the convex side of a wave (5). Both waving and the gravitropic response in roots are mediated by differential growth. This causes a reorientation of the root tip toward the gravity vector and results in root bending. We therefore tested whether changing the direction of root growth by rotating plants through an angle of 135° affects lateral root formation. We observed the initiation of a lateral root at the convex side of the gravity-induced curve [supporting information (SI) Fig. S1]. Auxin is a major regulator of lateral root development; the transcription of primary auxin response genes is among the first events of LRI (5). We therefore analyzed the formation of auxin-response maxima during gravitropic curvature. Here, the auxin-sensitive DR5::GUS reporter fusion marked sites of LRI (Fig. S1B). Before rotation, DR5-driven nuclear-localized YFP (pDR5rev::3XVENUS-N7) fluorescence in the distal elongation zone, was seen mainly in the protoxylem cell files, distributed equally between both poles (Fig. S2D). Five hours after rotation, the signal was detected in the epidermis and cortex of the concave side in the elongation zone as previously described (6). After the same time, an unequally distributed DR5 signal was observed also in the elongation zone, in protoxylem cells and predominantly in the xylem pole of the convex side of the root (Fig. 1B).
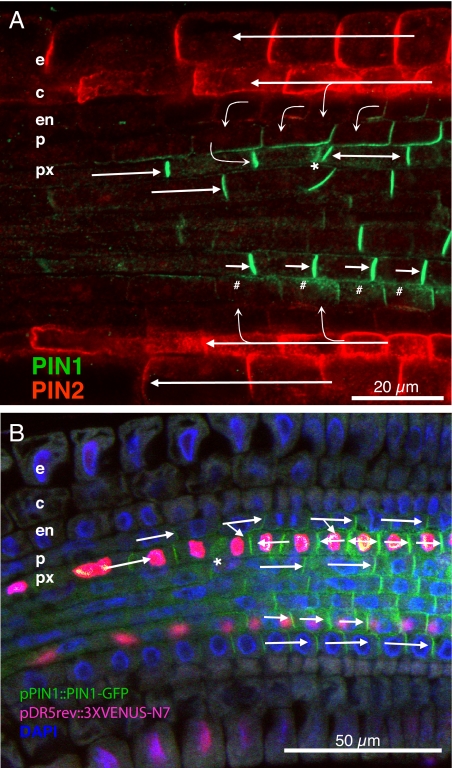
Fig. 1.
PIN protein localization in gravistimulated roots of wild type and pPIN1::PIN1- GFP, pDR5rev::3XVenus-N7 transgenic lines. (A) root elongation zone 3 h after gravistimulation. Immunolocalization with anti-PIN1(green) and anti-PIN2 (red). Epidermis (e), cortex (c), endodermis (en), pericycle (p), px (protoxylem), arrows indicate inferred auxin fluxes, asterisk (*) and hash (#) indicate diffuse cytosolic PIN1 localization. (B) PIN1-GFP (green) and DR5-Venus (pink), 5 h after gravistimulation, DAPI labeled nuclei (blue), asterisk (*) indicates protoxylem cell with diffuse PIN1-GFP signal.
We then tested whether a gravitropic response in the root meristem is necessary for LRI. We analyzed LRI in two agravitropic genotypes: aux1–7 and eir1–1, each lacking an auxin transport protein (7, 8). These proteins AUX1 and PIN2, are also required for proper lateral-root spacing. We observed that in both genotypes, when visible, lateral roots developed on the convex side of curves in 100% of roots analyzed (n = 75) (Fig. S3). This strict correlation was also seen when aux1–7 roots or eir1–1 roots formed loops (n = 75) (Fig. S3). We therefore conclude that lateral root positioning is consistent in relation to root shape, even in the absence of a gravitropic response.
LRI Is Induced by Transient Manual Bending.
To test the hypothesis that LRI is caused by mechanical changes to the root, a root-bending assay was developed. When 10-day-old roots were bent with fine forceps through 135° and immediately returned to their original position, we detected DR5-driven GUS activity at the site of the bend after five hours, on the side that was convex when bent (Fig. 2B). Lateral root emergence then proceeded normally at the site of bending, with a lateral root emerging after 72 hours (Fig. 2C). Seventy percent of roots (n = 45) responded in this way when bent within 1 mm of the root tip and only forty percent (n = 40) when bent 3 mm away from the root tip. Fifteen percent (n = 33) of unbent control roots developed a lateral root 1 mm behind the tip and only 5% did so further away (3 mm). These data demonstrated that the application of a mechanical force induces LRI. The fact that, after bending, lateral roots were never positioned on the concave side of the main root at the time of bending (in contrast to the regular left-right pattern observed in unbent roots) rules out the possibility that lateral initiation coincides with, but is independent from, root bending. Moreover, these data showed that the efficiency of LRI was dependent on distance from the root tip.
Fig. 2.
Lateral root primordia induced after manual curvature. (A-C), LRI in manually bent 10-day-old pDR5::GUS transgenic plants. (A) before bending, inset indicates root tip bent with fine forceps to 135° from its original position. (B) lateral root primordium as indicated by GUS signal (arrow) five hours after roots were bent with forceps to 135° and immediately returned. (C) Lateral root emergence after 72 h at the site of root bending (arrow).
Coordination of the Early Events of LRI.
To correlate DR5 expression with the expression of WOX5, a marker of the root quiescent center (9), pDR5::GFP plants (6) were subjected to a modified bending assay (Fig. 3A). Here, roots were bent 1 mm behind the tip until the tip touched the rest of the root and immediately released. Roots then settled unaided to an angle of between 30° and 90° with respect to their original position. As with the first bending method described above, LRI and development proceeded normally. In all root bending assays, the growth rate of the main was unaffected indicating that no significant damage was caused by the bending (Table S1). In 80% (n = 25) of the roots analyzed, we detected pDR5::GFP expression at between five and six hours after root bending on the convex side of the curved root, at the site of the newly initiated lateral root. A GFP signal was simultaneously observed in the protoxylem, in two cells of the adjacent pericycle (the cell layer from which lateral roots are formed) and in either two or three cells of the endodermis (Fig. 3A).
Fig. 3.
pDR5::GFP and pWOX5-GFP expression after root bending. (A) Five hours after root bending, DR5-GFP signal is present throughout the protoxylem (white arrows), except on the concave side of bends. DR5-GFP signal is detected on the convex side of the bend in the developing lateral root (yellow arrows). (B-E) six-day-old pWOX5::GFP transgenic plants bent and left to grow for between 5 h and 24 h. (Scale bar, 20 μm.)
The homeobox gene WOX5, is expressed in the quiescent center of the root tip and is required for stem cell maintenance in the root meristem (9). We constructed a pWOX5::GFP reporter and analyzed its expression in response to manual root bending. We detected WOX5 promoter activity in two pericycle cells at the site of LRI from five hours after a bending stimulus (Fig. 3 B-E). Auxin induces WOX5 expression (10). The simultaneous transcription from DR5 and WOX5 promoters in the pericycle suggests auxin acts very early to specify the quiescent center of the new lateral root.
Signals from the Shoot or Root Tip are Not Required for LRI.
In a two-phase model which has been used to explain lateral root formation during early seedling development, (i) lateral root primordium (LRP) initiation is dependent on an auxin source in the root tip, and (ii) LRP emergence is dependent on leaf-derived auxin (11).
Roots of control pDR5::GFP plants, with shoots and root tips intact, were subjected to the second bending assay (Fig. 4). After a recovery time of 24 h, a stage II primordium (after the first periclinal cell division, classification after ref. 12) was observed at the site of the bend (Fig. 4B); GFP fluorescence was detected exclusively at the convex side of the bent root in differentiating xylem cells, the lateral root primordium cells, and in the adjacent endodermis. After two days, 8% of bent regions had emerged lateral roots (Table S2).
Fig. 4.
The origin of auxin during LRI and development. Six-day-old pDR5::GFP plants with (A) root tips bent and left to grow for 24 h. (B) enlargement of boxed areas in A showing lateral root primordium (arrowheads). (C) lateral roots 120 h after root bending. (D) pDR5::GFP seedlings were cut at the root-shoot junction and the roots allowed to grow vertically for 24 h. (E) enlargement of boxed areas in D showing lateral root primordium (arrowheads). (F) No lateral root can be observed in plants described in D 120 h after root bending. (G) shoots intact and 24 h after the root tip was removed. (H) enlargement of boxed area in G showing lateral root primordium (arrowhead). (I) lateral roots 120 h after root bending. (Scale bar, 50 μm.)
To test whether a signal from the shoot is necessary for LRI, we subjected to bending pDR5::GFP plants 24 h after the removal of their aerial tissues (Fig. 4 D-F). After a further 24 h, GFP fluorescence was detected as described for the uncut root (Fig. 4E). However, lateral root primordium development was arrested at stage I, the stage at which the first asymmetric anticlinal cell division is observed (Fig. 4E, Table S2). Subsequently, the root primordia did not develop, even after a further five days (Fig. 4F). However, when transferred onto growth medium supplemented with 1 μM IAA, plants first developed a single lateral root at the convex side of the bend in every case (Fig. S4H).These results prove that auxin from the shoot is not necessary for LRI by mechanical stress, but is required for subsequent primordium outgrowth, consistent with results recently reported elsewhere (13).
To test whether the basipetal transport of solutes from the root tip could regulate LRI after root bending, seedlings were bent after the removal of their root apical meristems (Fig. 4 G-I). After 24 h, this resulted in a larger number of lateral root primordia being initiated between the cut and the site of mechanical bending (Fig. 4 G and I). Furthermore, these primordia developed more rapidly than lateral roots of plants with intact root tips, reaching stage III (a primordium of three cell layers) after 24 h (Fig. 4H). After two days, 100% of bent regions had emerged lateral roots (Table S2). Therefore, the root tip had an inhibitory effect on the initiation and subsequent development of lateral root primordia after bending.
Lateral root development is disrupted by increased cytokinin concentration (14). However, it is unlikely that cytokinins form this tip-derived inhibitory signal. Bending a root with its tip removed in presence of 100 nM N-benzyladenine (6-BA), a cytokinin concentration known to inhibit lateral root development (14), did not influence the number, the position or the frequency of initiation events (Fig. S5).
To test whether this increase in lateral root number could have been caused by the removal of the major auxin sink following excision of the root apex and subsequent accumulation of auxin in the distal elongation zone, we removed both the shoot and the root tip of DR5::GUS plants, using the previously described treatments as controls. In this case, lateral roots were initiated and arrested at either stage II or stage III (Fig. S4D, Table S2); LRI therefore proceeded in a similar manner in the absence of an aerial auxin source, or a root tip-driven auxin sink. When repeated in the presence of 1 μM IAA, lateral roots proliferated, but never emerged on the concave side of bends (Fig. S4K). These results indicate that a balance between auxin from the shoot and an as yet unidentified signal from the root tip controls LRI and emergence.
The Role of Auxin Transporters in LRI.
It has been shown that lateral roots of the aux1 genotype are formed in an unequal left-right distribution along the main root and do not develop on every curve (5), with nine-day-old aux1-7 seedlings forming approximately half the number of lateral root primordia than wild type (WT); aux1-7 LRP also display a developmental delay in the transition from stage I to stage II (15). These data are consistent with our observation that a pAUX1::AUX1-YFP reporter is only induced in pericycle cells at the site of LRI seven hours after a bending stimulus (at the time a stage I LRP is formed), supporting the hypothesis that AUX1 is not directly involved in the first events of LRI but in the development of stage I primordia (Fig. S6). That aux1 seedlings form less LRP in total could be related to a permissive function of a background expression of AUX1 observed in the vasculature of the region in which lateral roots are formed or to other associated functions, for example in post-phloem unloading in the root tip (16) or auxin loading in shoot tissues (15) (Fig. S6).
We observed that lateral root induction by mechanical stress was also less efficient in both eir1- 1 and aux1–7 when compared to WT; approximately half of the aux1–7 and eir1–1 seedlings developed lateral roots (Table S3). To test whether abnormal auxin transport from the root apical meristem of aux1–7 or eir1–1 precludes normal lateral root distribution, we removed the root tip in these genotypes. In this case, both mutants developed lateral roots in response to bending with an increased frequency (Table S3). We therefore conclude that LRI by mechanical stress is independent of AUX1 and PIN2, but the root tip is necessary for subsequent lateral root outgrowth.
We analyzed DR5-dependent gene expression to visualize lateral root primordia in the eir1–1 genotype (Fig. S4 I and J). After bending intact eir1–1DR5::GUS roots, all displayed lateral root primordia at the expected position on the convex side of curves, proving that LRI per se is not dependent on the action of PIN2 (Fig. S4 I and J). Taken together, these results suggest that LRI is a direct consequence of root bending. These data lead us to hypothesize that a physical strain caused by either manual root bending or gravitropic curvature initiates a lateral root.
PIN Proteins Direct Auxin Flux to the Site of LRI.
Both the gravitropic response and manual bending result in an asymmetric distribution of DR5-dependent transcription in differentiating xylem cells, but in different regions of the root (Fig. S1 and Fig. 2). However, unlike younger vasculature in the elongation zone, the xylem cells affected by the manual bending assay do not express an important auxin-transport related protein, PIN1. We therefore used the more physiologically relevant gravitropic induction of LRI to examine local auxin transport dynamics shortly before the initiation of lateral root primordia.
First, we inspected root tips (n = 30) of straight-grown four-day-old seedlings (Fig. S2). PIN1 and PIN2 localization were used to indicate the direction of auxin flux (17). Throughout the stele, and in the endodermis and cortex of the meristematic zone, PIN1 was localized exclusively to the basal (lower) membrane of cells (17) (Fig. S2A). In the elongation zone (as defined by the slightly bigger size of the cells), PIN1 was absent from the cortex. Here, PIN1 was localized in the basal membrane of endodermal cells (Fig. S2A). In the pericycle cell file of the elongation zone, PIN1 was localized both basally and laterally toward provascular cell file. In the meristematic zone, PIN2 was localized apically (at the top of cells) in epidermis and lateral root cap cells, and co-localized with PIN1 in the basal (lower) membrane of cortex cells forming a previously reported loop of auxin flow (17) (Fig. S2B). PIN2 was absent from the endodermis of the meristematic zone. Further from the root tip but still in the meristematic zone, PIN2 was localized apically in the epidermis, directing auxin toward the distal elongation zone. Between the meristematic and elongation zones, PIN2 was localized to both the apical and basal side of one, and sometimes two cortical cells. In the epidermis and cortex of the elongation zone, PIN2 was localized increasingly apically as the distance from the root tip increased. In the endodermis of the elongation zone, PIN2 was localized laterally toward the stele in four or five cells (Fig. S2B). In the stele, PIN2 was entirely absent.
These data revealed the presence of an as yet unreported loop of auxin flux in 100% of the plants, with PIN2 and PIN1 directing auxin toward the stele in the lower part of the elongation zone through the endodermis and pericycle respectively (Fig. S2C). In accordance with these observations, auxin (as visualized by a DR5 reporter fusion) is localized to both protoxylem cell files throughout the root tip (Fig. S2D).
The presence of this PIN-dependent route by which auxin can be circulated to the elongation zone is potentially significant for the recently proposed genetically preprogrammed “priming” of pericycle cells necessary for LRI (5), and should be taken into account when designing future mathematical models to predict PIN-dependent auxin flux in the root tip.
PIN1 Relocalizes at the Site of LRI upon Gravitropic Curvature.
Three hours after plants were rotated through 135°, significant changes in PIN1 localization in the root tip were observed. PIN1 was seen on both the apical and basal side of a single protoxylem cell on the convex side of the of the elongation zone after 3 h in 70% (n = 25) of the seedlings, (Fig. 1A [double-headed arrow], Fig. S7) and was present on only the apical side of between 1 and 3 basipetal protoxylem cells on the convex side of the curving root after 5 h (Fig. 1B, Fig. S7). In these protoxylem cells, the gravity-induced change in PIN1 subcellular localization preceded by 2 h the asymmetrical distribution of DR5-driven nuclear-localized YFP (pDR5rev::3XVENUS-N7) fluorescence in the protoxylem poles of the bending region (Fig. 1B) compared to the unbent control (Fig. S2D). These data are consistent with a relocalization of PIN1 in response to gravitropic curvature serving to maintain locally auxin concentration in a specific developing xylem cell. PIN1 has been shown previously to mediate lateral root emergence (2). The relocalization reported here therefore represents a second, earlier mechanism by which PIN1 might influence lateral root development. Three hours after bending, in the protoxylem on the convex side of the curve, a diffuse cytosolic PIN1 localization was observed to be restricted to the cell immediately above that showing PIN1 on both sides (Fig. 1A). After 5 h, PIN1 was lost entirely from the protoxylem cell in which this diffuse cytosolic localization was observed (Fig. 1B [asterisk]).
Changes in PIN1 localization in the pericycle in response to gravitropic curvature are restricted to the concave side of bends. Here, a similar diffuse cytosolic PIN1 signal was observed, but close to the lateral wall in contact with the vasculature (Fig. 1A [hash]). This change in subcellular localization corresponded to a relatively weak pDR5rev::3XVENUS-N7 signal (Fig. 1B) in adjacent vascular cells when compared to similar cells on the convex side of the root.
These data suggest that gravitropic root curvature initiates positional cues in the protoxylem cell file which determine the site of LRI. These cues act on an endogenous signaling cascade which changes the subcellular localization of PIN1 in the elongation zone. An investigation into decreased lateral root formation in weak alleles of the GNOM gene (which encodes an ARF-GEF necessary for PIN1 function) predicted that a relocalization of PIN1 in the pericycle would be necessary for LRI (18). Although in our studies, no relocalization was observed in the pericycle before the accumulation of a DR5-dependent signal, we suggest that a similar function can be attributed to PIN1 relocalization in the subtending protoxylem cell file. In contrast to the gnom phenotype, overexpression of PIN1 in 35S::PIN1 plants initiates more primordia (2), suggesting that PIN1 is important for LRI. However, plants of the pin1 genotype initiate no fewer lateral roots than WT plants. This suggests a degree of functional redundancy among PIN proteins in lateral root development.
The relocalization of PIN1 upon bending in protoxylem cells is predicted to focus auxin into the basipetal neighboring protoxylem cell. In the adjacent pericycle cells, PIN1 is localized to the lateral membrane; also directing auxin into the same single protoxylem cell and further increasing the auxin concentration therein. An increase in auxin concentration would be expected to inhibit the internalization of PIN1 (19). However, in this cell (into which PIN1 focuses auxin flux and is therefore inferred to contain an auxin concentration maximum), PIN1 becomes internalized. This unexpected response could be a specific adaptation for LRI.
We then tested whether gravity-induced PIN1 relocalization in the protoxylem is dependent on auxin signaling. ARFs are transcriptional regulators of early auxin response genes (20). The arf7arf19 double mutant is severely impaired in lateral root formation, a phenotype not observed in arf7 and arf19 single mutants, indicating that lateral root formation is redundantly regulated by ARF7 and ARF19 (21). Arf7/19 double knockout plants (nph4–1arf19–1) were rotated through 135°. At the convex side of the induced bend, PIN1 was observed at the apical and basal side of a single protoxylem cell after 5 h in 7 of 8 seedlings analyzed (Fig. S2E–G). No other PIN1 relocalization was observed. Nph4–1arf19 plants are mildly agravitropic, explaining why PIN1 relocalization occurred after 5 h, as compared to after only 3 h in WT plants. We therefore conclude that the first stage of LRI, a gravity-induced PIN1 relocalization in cells of the elongation zone, is independent of ARF7 and ARF19. These transcription factors are therefore necessary for later stages of lateral root development. The LRI signaling events before the relocalization of PIN1 are unknown. However, several candidate signaling components can be suggested. For example, cortical microtubule reorganization and mechanosensory ion channels could relay physical perturbations into a physiological response (22).
Root Bending Induces Lateral Roots in Auxin-Insensitive Lateral Root-Deficient Genotypes.
After its inferred accumulation at the site of an incipient lateral root primordium, auxin induces gene transcription to trigger lateral root growth. As the first stages of LRI are independent of ARF7 and ARF19, we examined at which stage of lateral root development these proteins are involved. We bent roots of two classes of auxin signaling mutants: aux/iaa and arf. The gain-of-function mutant solitary root is impaired in its gravitropic response, synthesizing a stabilized form of IAA14, an Aux/IAA-family negative regulator of auxin signaling (23). This mutation also prevents the formation of lateral roots and root hairs (Fig. S8A). Manual bending induces a lateral root in the slr-1 mutant background in 10% of cases (Fig. S8 B and C). These lateral roots are initiated in an identical manner to the WT after manual bending. Root hairs are also initiated in slr-1 upon bending. However, they do not develop normally and form densely packed balloon-like structures (Fig. S8D).
To infer the expression pattern of IAA14 in mechanically stimulated roots, we stained pIAA14::GUS plants. In unbent plants, we observed GUS activity in the lateral root cap and in epidermal cells of the distal elongation zone (Fig. S8E). Upon manual bending 1.5 mm from the root tip, GUS activity was retained in epidermal cells on the concave side of the bend and detected in pericycle cells in contact with the xylem pole on the convex side of the root at the site of bending (Fig. S8H). Activity of the IAA14 promoter was subsequently down-regulated upon the first cell divisions in the pericycle (Fig. S8I).
We tested whether lateral root development after manual bending involves auxin-induced ARF7/ARF19-mediated transcription. Two independent lines of arf7arf19 double mutants, msg1–2arf19–1 and nph4–1arf19–1, were used and gave similar results. When six-day-old seedlings were bent manually, 96.6% of WT plants developed lateral roots at the convex side after three days; almost no lateral root was observed in either arf7arf19 double mutant (Fig. S9 A–C, Table S4). However, when, after complete recovery, the root tips of the same seedlings were removed, and roots were again bent and grown for a further 96 h, 50% of arf7arf19 mutant plants developed a lateral root at the convex side of the bend (Fig. S9 D and E; Tables S4 and S5). Significantly, a lateral root also then emerged from the initial bend, suggesting a primordium had been initiated but failed to emerge. This result supports the hypothesis that specific pericycle cells can be marked as sites of LRI upstream of auxin signaling.
The specific degradation of Aux/IAAs in pericycle cells is necessary for LRI (3). This degradation is a result of auxin accumulation in the same cells (5). Auxin accumulation therefore defines the site of LRI, but the mechanisms involved in the selection of this site remain unclear. Here we show that IAA14 is expressed at the site of LRI in pericycle cells in response to an auxin maximum generated by manual bending, and subsequently disappears from dividing lateral root founder cells. We therefore propose that mechanical strain, caused either by gravitropic curvature or by the application of an external force, determines the site of LRI before auxin accumulation.
In the case of gravitropic curvature, auxin accumulation in the pericycle follows a reorientation of PIN1 in the subtending protoxylem cell file. No accompanying lateral reorientation of PIN1 in protoxylem cells was observed. We therefore hypothesize that if the lateral movement of auxin from the protoxylem to the pericycle cell file is significant, it happens either by diffusion or by the specific action of another auxin transport protein. In the case of manual bending, no PIN1 relocalization was observed as it is not expressed in the bent portion of the root tip. However, the fact that a transient bending of the root is sufficient to induce LRI, and that the rate of root growth did not change, suggests that a signaling cascade is initiated by root bending.
Auxin does not induce lateral roots in slr1 at physiologically relevant concentrations (24), therefore the targeted degradation of specific Aux/IAA proteins mediate auxin's ability to promote lateral root development. However, nuclear auxin signaling through the degradation of IAA14 is not strictly necessary for lateral root formation, as removing the root tip of slr1 plants causes a limited number of lateral roots to appear (23). Similarly, we have shown that if arf7/19 double knock-out plants are subjected to mechanical strain, either by manual bending or by gravitropic curvature, a lateral root is initiated, but does not develop. Additional evidence that the earliest stages of LRI are independent of Aux/IAAs degradation comes from the observation that nuclei of adjacent pericycle cells move toward each other (an early event of LRI) when IAA17 is stabilized in the pericycle (5). We therefore hypothesize that a second, auxin-independent lateral root-promoting signal either moves from the protoxylem or is generated directly in specific pericycle cells in response to a mechanical stimulus. The fact that lateral roots form upon removal of the root tip in both arf 7/19 and slr1 genotypes suggests that this second signal acts downstream of ARF-dependent gene transcription and is inhibited by a third, mobile signal from the root tip. In this model, subsequent removal of the root tip would release a brake on the development of quiescent, lateral root-marked pericycle cells, resulting in the development of a lateral root. This mobile inhibitory signal originating in the root tip possibly acts to suppress downstream signaling in lateral root formation, for example via regulation of LBD gene function (4). This auxin-independent signal could mediate the ability of a main root to suppress the growth of lateral roots and influence apical dominance in the root.
The evolutionary relationship between these distinct mechanisms of lateral root regulation (as well as the exact nature of auxin-independent signals) and their relative developmental significance in different species are all areas for further study.
Conclusions
Our data demonstrate that bending causes the initiation of lateral root primordia. Here, we report the earliest observable event of LRI after gravitropic curvature to be a changing polar PIN position in differentiating xylem cells. PIN1 relocalization is followed by auxin-dependent gene expression in the protoxylem. The signaling events between the bending stimulus and PIN1 relocalization are unknown. We hypothesize that these signals are auxin-independent and act on cell polarity. Two further auxin-independent signals affect LRI, both acting downstream of ARF7/19-mediated transcription in the protoxylem pericycle cell file. Removing root tips after mechanically bending arf7/19 double mutants demonstrates that the first is able to promote LRI, and acts antagonistically to the second, a root tip-derived inhibitory signal.
In nature, LRI is closely integrated with a range of environmental stimuli such as water availability, nutrients, pathogens, and symbionts. All of these factors, in combination with the plant's genetic background, influence the shape of the root system, making the control of root architecture an ideal model to study the environmental control of plant development. It is not yet clear whether an ability to develop lateral roots upon bending of the main root confers a selective advantage. But it is tempting to speculate that the efficient exploration of the root's environment or increased anchorage of the plant could be facilitated by such a response.
Materials and Methods
Plant Material and Growth Conditions.
A. thaliana (L.) Heynh. ecotype Columbia (Col-0) and ecotype WS; pDR5::GFP (6), pDR5::GUS (24), pIAA14::GUS (a gift from H. Fukaki, Graduate School of Science, Kobe University, Kobe, Japan; 3); slr-1 (23); pin2/eir1–1 (8); pin2pDR5::GUS (25); pPIN1::GFP (2); pDR5rev::3XVENUS-N7 (a gift from M. Heisler, California Institute of Technology, Pasadena, CA; ref. 26); nph4–1arf19–1 (obtained from NASC, N24625), msg1–2arf19–1 (obtained from NASC, N24627); pWOX5::GFP was constructed as follows: a 4.46 kb 5′ upstream promoter region of WOX5 was amplified from WT (Col-0) genomic DNA and cloned into pGEM-T, producing AKS49. For WOX-erGFP, the WOX5 promoter region was isolated from AKS49 and cloned in a pGreenII-derived vector, producing WOX5:Gal4VP16-UAS:erGFP (27, 28); Seeds were surface-sterilized and sown on solid Arabidopsis medium (AM) (2.3 g/liter MS salts, 1% sucrose, 1.6% agar–agar (pH 6.0) adjusted with KOH). After vernalization for 2 days at 4 °C, seeds were germinated under long-day period (16 h light, 8 h darkness).
Hormone Treatment.
Six-day-old pPIN::1GFP plants, grown on solid AM (described above), were transferred to solid AM supplemented or not with 1 μM IAA or 100 nM N-benzyladenine. After transfer, plants were grown for 48 h or 72 h. Root tips were then removed at an average of 400 μm behind the tip.
Immunolocalization.
Six-day-old seedlings were rotated through 135° for 5 h, then fixed with 4% paraformaldehyde in PBS (pH 7.3) and used for whole-mount in situ immunolocalization according to (29).
Microscopy.
Histological detection of β-glucuronidase (GUS) activity was performed according to (30). Plants were mounted in chloral hydrate:glycerol:water (8:3:1, w/vol/vol). Plants containing fluorescent markers were fixed with 4% formaldehyde and mounted in Prolong Gold antifade reagent containing DAPI (Molecular Probes). For light microscopy samples were observed with a Zeiss Axiovert 200M MOT (Carl Zeiss MicroImaging) for high magnification pictures. Low magnification views were taken with a Zeiss Stemi SV11 Apo stereomicroscope (Carl Zeiss MicroImaging), viewed under differential interference contrast (DIC) optics.
Fluorescent proteins were analyzed with a Zeiss LSM 5 DUO scanning microscope. To monitor GFP and DAPI fluorescence, we used multitracking in frame mode. GFP was excited using the 488 nm laser line in conjunction with a 505–530 band-pass filter. DAPI was excited with the 405 nm laser line and collected using a 420–480 nm band-pass filter. For VENUS with GFP, both fluorescent proteins were excited using the 458 nm laser line, and the emission was separated using the on-line unmixing feature of the Meta spectral analyzer. Images were analyzed with the LSM image browser (Carl Zeiss MicroImaging) and representative of at least 20 individual plants.
Supplementary Material
Acknowledgments.
We thank Prof. Peter Schopfer and members of our team for helpful comments on the manuscript. We also gratefully acknowledge the excellent technical support from Katja Rapp and Bernd Gross. This work was supported by the Collaborative Research Center 592, the Excellence Initiative of the German Federal and State Governments (EXC 294), Graduierten kolleg 1305, European Space Agency, Bundesministerium für Forschung und Technik (BMBF), and Deutsches Zentrum für Luft und Raumfahrt.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0807814105/DCSupplemental.
References
- 1.De Smet I, Vanneste S, Inzé D, Beeckman T. LRI or the birth of a new meristem. Plant Mol Biol. 2006;60:871–87. doi: 10.1007/s11103-005-4547-2. [DOI] [PubMed] [Google Scholar]
- 2.Benková E, et al. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/s0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 3.Fukaki H, Nakao Y, Okushima Y, Theologis A, Tasaka M. Tissue-specific expression of stabilized SOLITARY-ROOT/IAA14 alters lateral root development in Arabidopsis. Plant J. 2005;44:382–395. doi: 10.1111/j.1365-313X.2005.02537.x. [DOI] [PubMed] [Google Scholar]
- 4.Okushima Y, Fukaki H, Onoda M, Theologis A, Tasaka M. ARF7 and ARF19 regulate lateral root formation via direct activation of LBD/ASL genes in Arabidopsis. Plant Cell. 2007;19:118–130. doi: 10.1105/tpc.106.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Smet I, et al. Auxin-dependent regulation of lateral root positioning in the basal meristem of Arabidopsis. Development. 2007;134:681–690. doi: 10.1242/dev.02753. [DOI] [PubMed] [Google Scholar]
- 6.Ottenschläger I, et al. Gravity-regulated differential auxin transport from columella to lateral root cap cells. Proc Natl Acad Sci USA. 2003;100:2987–2991. doi: 10.1073/pnas.0437936100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pickett FB, Wilson AK, Estelle M. The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol. 1990;94:1462–1466. doi: 10.1104/pp.94.3.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roman G, Lubarsky B, Kieber JJ, Rothenberg M, Ecker JR. Genetic analysis of ethylene signal transduction in Arabidopsis thaliana: Five novel mutant loci integrated into a stress response pathway. Genetics. 1995;139:1393–1409. doi: 10.1093/genetics/139.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarkar AK, et al. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–814. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- 10.Gonzali S, et al. A turanose-insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana. Plant J. 2005;44:633–45. doi: 10.1111/j.1365-313X.2005.02555.x. [DOI] [PubMed] [Google Scholar]
- 11.Bhalerao RP, et al. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002;29:325–332. doi: 10.1046/j.0960-7412.2001.01217.x. [DOI] [PubMed] [Google Scholar]
- 12.Malamy JE, Benfey PN. Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development. 1997;124:33–44. doi: 10.1242/dev.124.1.33. [DOI] [PubMed] [Google Scholar]
- 13.Swarup K, et al. The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol. 10:946–954. doi: 10.1038/ncb1754. [DOI] [PubMed] [Google Scholar]
- 14.Li X, Mo X, Shou H, Wu P. Cytokinin-mediated cell cycling arrest of pericycle founder cells in LRI of Arabidopsis. Plant Cell Physiol. 2006;47:1112–1123. doi: 10.1093/pcp/pcj082. [DOI] [PubMed] [Google Scholar]
- 15.Marchant A, et al. AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell. 2002;14:589–597. doi: 10.1105/tpc.010354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Swarup R, et al. Localisation of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex. Genes Dev. 2001;15:2648–2653. doi: 10.1101/gad.210501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blilou I, et al. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 18.Geldner N. The plant endosomal system—its structure and role in signal transduction and plant development. Planta. 2004;219:547–560. doi: 10.1007/s00425-004-1302-x. [DOI] [PubMed] [Google Scholar]
- 19.Paciorek T, et al. Auxin inhibits endocytosis and promotes its own efflux from cells. Nature. 2005;435:1251–1256. doi: 10.1038/nature03633. [DOI] [PubMed] [Google Scholar]
- 20.Guilfoyle TJ, Hagen G. Auxin response factors. Curr Opin Plant Biol. 2007;10:453, 460. doi: 10.1016/j.pbi.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 21.Wilmoth JC, et al. NPH4/ARF7 and ARF19 promote leaf expansion and auxin induced lateral root formation. Plant J. 2005;43:118–130. doi: 10.1111/j.1365-313X.2005.02432.x. [DOI] [PubMed] [Google Scholar]
- 22.Fischer K, Schopfer P. Physical strain-mediated microtubule reorientation in the epidermis of gravitropically or phototropically stimulated maize coleoptiles. Plant J. 1998;15:119–123. doi: 10.1046/j.1365-313x.1998.00173.x. [DOI] [PubMed] [Google Scholar]
- 23.Fukaki H, Tameda S, Masuda H, Tasaka M. Lateral root formation is blocked by a gain-of-function mutation in the SOLITARY-ROOT/IAA14 gene of. Arabidopsis. Plant J. 2002;29:153–168. doi: 10.1046/j.0960-7412.2001.01201.x. [DOI] [PubMed] [Google Scholar]
- 24.Ulmasov T, Murfett J, Hagen G, Guilfoyle TJ. Aux/IAA proteins repress expression of reporter genes containing natural and highly active synthetic auxin response elements. Plant Cell. 1997;9:1963–1971. doi: 10.1105/tpc.9.11.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabatini S, et al. An auxin-dependent distal organizer of pattern and polarity in the Arabidopsis root. Cell. 1999;99:463–472. doi: 10.1016/s0092-8674(00)81535-4. [DOI] [PubMed] [Google Scholar]
- 26.Heisler MG, et al. Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol. 2005;15:1899–1911. doi: 10.1016/j.cub.2005.09.052. [DOI] [PubMed] [Google Scholar]
- 27.Hellens RP, Edwards EA, Leyland NR, Bean S, Mullineaux PM. pGreen: A versatile and flexible binary Ti vector for Agrobacterium-mediated plant transformation. Plant Mol Biol. 2000;42:819–832. doi: 10.1023/a:1006496308160. [DOI] [PubMed] [Google Scholar]
- 28.Sabatini S, Heidstra R, Wildwater M, Scheres B. SCARECROW is involved in positioning the stem cell niche in the Arabidopsis root meristem. Genes Dev. 2003;17:354–358. doi: 10.1101/gad.252503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Friml J, et al. A PINOID-dependent binary switch in apical-basal PIN polar targeting directs auxin efflux. Science. 2004;306:862–865. doi: 10.1126/science.1100618. [DOI] [PubMed] [Google Scholar]
- 30.Scarpella E, Francis P, Berleth T. Stage-specific markers define early steps of procambium development in Arabidopsis leaves and correlate termination of vein formation with mesophyll differentiation. Development. 2004;131:3445–3455. doi: 10.1242/dev.01182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.