Abstract
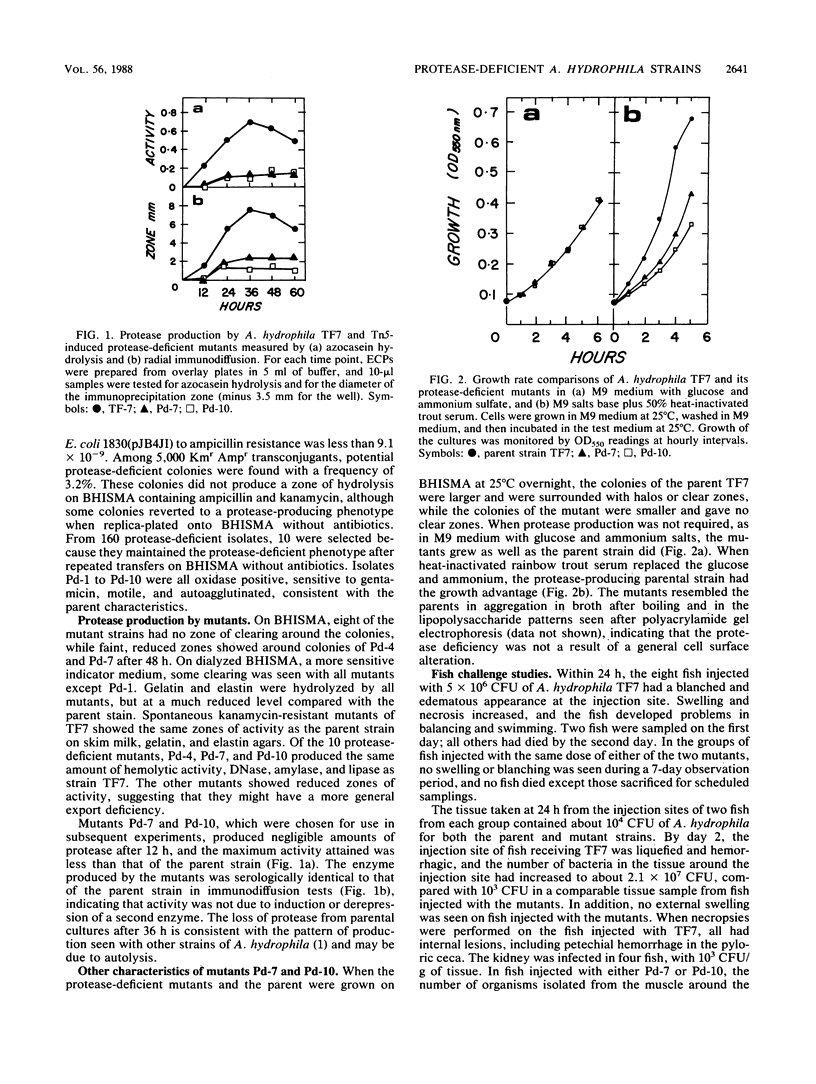
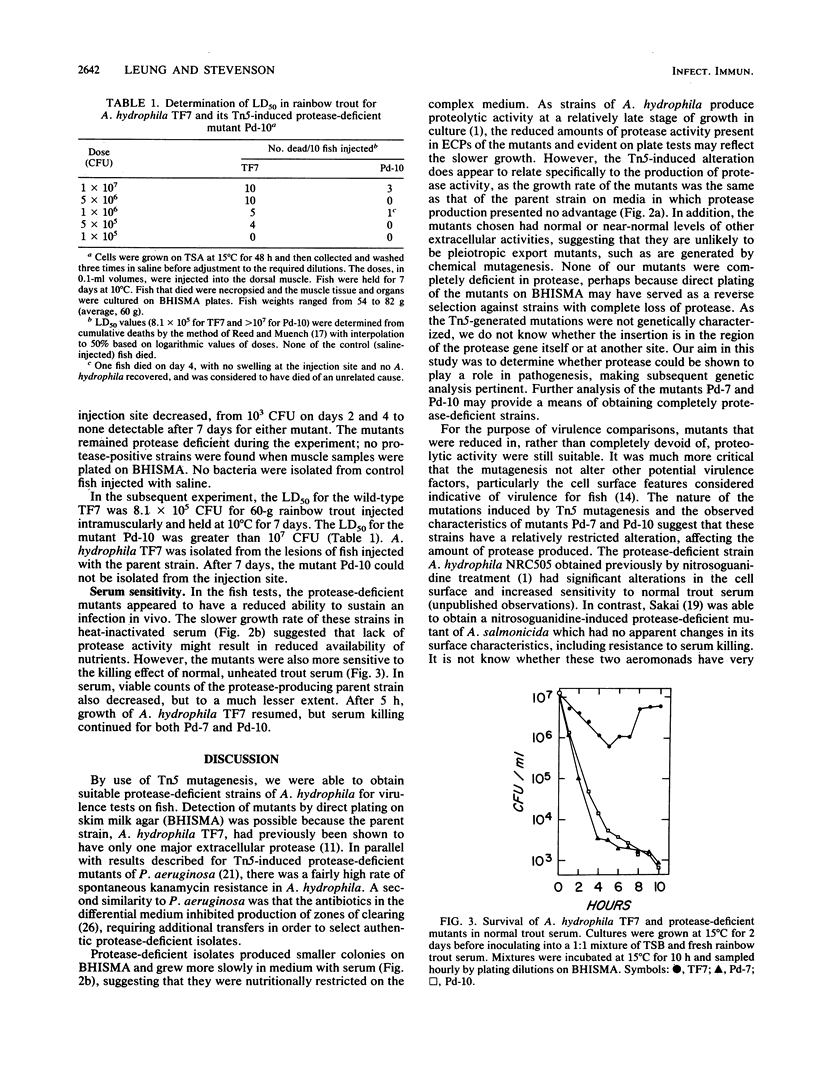
Protease-deficient strains of Aeromonas hydrophila TF7 were induced by transposon Tn5 mutagenesis, with Escherichia coli 1830(pJB4JI) as the Tn5 donor. The parent strain has the cell surface characteristics associated with virulence for fish, and as it produces a single metalloprotease, mutants could be distinguished by direct plating on brain-heart infusion skim milk agar. Mutants Pd-7 and Pd-10 still produced metalloprotease, but at reduced levels and only after prolonged incubation. The activities of other exoenzymes and hemolysin were unaffected, and the mutants autoagglutinated in broth, indicating that the cell surface characteristics of A. hydrophila TF7 had been retained. Unlike the parent strain, the mutants did not produce lesions or mortalities in rainbow trout (Salmo gairneri) when 5 X 10(6) CFU were injected intramuscularly. The bacterial cells were completely cleared from the site of the injection and the organs within 7 days. For 60-g rainbow trout held at 10 degrees C, the 50% lethal dose of Pd-10 was greater than 10(7) CFU, compared with 8.1 X 10(5) CFU for the parent strain. The mutants were significantly more susceptible than the parent strain to the bactericidal effect of fresh normal trout serum in vitro. Mutants Pd-7 and Pd-10 grew as well as the parent on M9 salts-glucose medium but more slowly on heat-inactivated fish serum. Thus, protease appears to be able to contribute to the establishment of A. hydrophila infection in fish both by overcoming initial host defenses and by providing nutrients for cell proliferation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan B. J., Stevenson R. M. Extracellular virulence factors of Aeromonas hydrophila in fish infections. Can J Microbiol. 1981 Oct;27(10):1114–1122. doi: 10.1139/m81-174. [DOI] [PubMed] [Google Scholar]
- Belland R. J., Trust T. J. Synthesis, export, and assembly of Aeromonas salmonicida A-layer analyzed by transposon mutagenesis. J Bacteriol. 1985 Sep;163(3):877–881. doi: 10.1128/jb.163.3.877-881.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooley J. S., Lallier R., Trust T. J. Surface antigens of virulent strains of Aeromonas hydrophila. Vet Immunol Immunopathol. 1986 Jun;12(1-4):339–344. doi: 10.1016/0165-2427(86)90138-8. [DOI] [PubMed] [Google Scholar]
- Hitchcock P. J., Brown T. M. Morphological heterogeneity among Salmonella lipopolysaccharide chemotypes in silver-stained polyacrylamide gels. J Bacteriol. 1983 Apr;154(1):269–277. doi: 10.1128/jb.154.1.269-277.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Activation of the hole-forming toxin aerolysin by extracellular processing. J Bacteriol. 1985 Jul;163(1):336–340. doi: 10.1128/jb.163.1.336-340.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard S. P., Buckley J. T. Intracellular accumulation of extracellular proteins by pleiotropic export mutants of Aeromonas hydrophila. J Bacteriol. 1983 Apr;154(1):413–418. doi: 10.1128/jb.154.1.413-418.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungh A., Wadström T. Aeromonas toxins. Pharmacol Ther. 1981;15(3):339–354. doi: 10.1016/0163-7258(81)90049-8. [DOI] [PubMed] [Google Scholar]
- Mittal K. R., Lalonde G., Leblanc D., Olivier G., Lallier R. Aeromonas hydrophila in rainbow trout: relation between virulence and surface characteristics. Can J Microbiol. 1980 Dec;26(12):1501–1503. doi: 10.1139/m80-248. [DOI] [PubMed] [Google Scholar]
- Nieto T. P., Ellis A. E. Characterization of extracellular metallo- and serine-proteases of Aeromonas hydrophila strain B51. J Gen Microbiol. 1986 Jul;132(7):1975–1979. doi: 10.1099/00221287-132-7-1975. [DOI] [PubMed] [Google Scholar]
- Ruangpan L., Kitao T., Yoshida T. Protective efficacy of Aeromonas hydrophila vaccines in nile tilapia. Vet Immunol Immunopathol. 1986 Jun;12(1-4):345–350. doi: 10.1016/0165-2427(86)90139-x. [DOI] [PubMed] [Google Scholar]
- Sakai D. K. Loss of virulence in a protease-deficient mutant of Aeromonas salmonicida. Infect Immun. 1985 Apr;48(1):146–152. doi: 10.1128/iai.48.1.146-152.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol P. A., Ohman D. E., Iglewski B. H. A more sensitive plate assay for detection of protease production by Pseudomanas aeruginosa. J Clin Microbiol. 1979 Apr;9(4):538–540. doi: 10.1128/jcm.9.4.538-540.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton M. J., Jagger K. S., Warren R. L. Transposon mutagenesis of Pseudomonas aeruginosa exoprotease genes. J Bacteriol. 1984 Jan;157(1):7–12. doi: 10.1128/jb.157.1.7-12.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Klotz F. W., Miller L. H. Pathogenesis of infectious diseases of fish. Annu Rev Microbiol. 1986;40:479–502. doi: 10.1146/annurev.mi.40.100186.002403. [DOI] [PubMed] [Google Scholar]
- Warren R. L., Baker N. R., Johnson J., Stapleton M. J. Selective inhibition of the accumulation of extracellular proteases of Pseudomonas aeruginosa by gentamicin and tobramycin. Antimicrob Agents Chemother. 1985 Apr;27(4):468–472. doi: 10.1128/aac.27.4.468. [DOI] [PMC free article] [PubMed] [Google Scholar]


