Abstract
Transforming growth factor-β (TGF-β) activity is controlled at many levels including the conversion of the latent secreted form to its active state. TGF-β is often released as part of an inactive tripartite complex consisting of TGF-β, the TGF-β propeptide, and a molecule of latent TGF-β binding protein (LTBP). The interaction of TGF-β and its cleaved propeptide renders the growth factor latent, and the liberation of TGF-β from this state is crucial for signaling. To examine the contribution of LTBP to TGF-β function, we generated mice in which the cysteines that link the propeptide to LTBP were mutated to serines, thereby blocking covalent association. Tgfb1C33S/C33S mice had multiorgan inflammation, lack of skin Langerhans cells (LC), and a shortened lifespan, consistent with decreased TGF-β1 levels. However, the inflammatory response and decreased lifespan were not as severe as observed with Tgfb1−/− animals. Tgfb1C33S/C33S mice exhibited decreased levels of active TGF-β1, decreased TGF-β signaling, and tumors of the stomach, rectum, and anus. These data suggest that the association of LTBP with the latent TGF-β complex is important for proper TGF-β1 function and that Tgfb1C33S/C33S mice are hypomorphs for active TGF-β1. Moreover, although mechanisms exist to activate latent TGF-β1 in the absence of LTBP, these mechanisms are not as efficient as those that use the latent complex containing LTBP.
Keywords: TGF-β activation
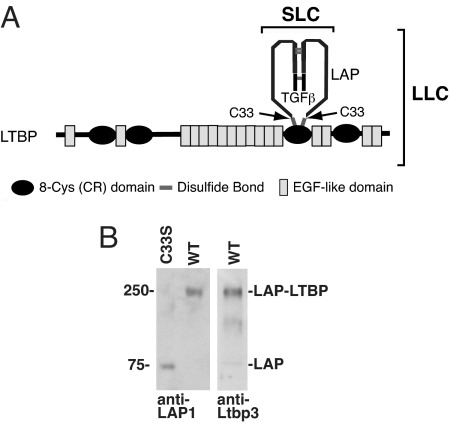
Transforming growth factor (TGF)-β1 is secreted as part of an inactive tripartite complex consisting of the 25-kDa TGF-β1 homodimer, the TGF-β1 dimeric propeptide, and a molecule of the latent TGF-β binding protein (LTBP) (Fig. 1A) (1, 2). TGF-β is cleaved from its initial translation product by intracellular proteolytic processing, but the liberated N-terminal propeptide remains associated with TGF-β by noncovalent bonds even after secretion. The union of TGF-β and its propeptide renders the growth factor latent. Thus, the TGF-β propeptide is called the latency-associated protein (LAP). LAP and LTBP are linked by disulfide bonds that form intracellularly between the cysteine 33 residues in each of the two LAP chains and a pair of cysteine residues in the LTBP. Four LTBPs, 1, 2, 3, and 4, are known, but only LTBP-1, -3, and -4 bind LAP (3). The complex of TGF-β1, LAP, and LTBP is called the large latent complex (LLC), whereas the complex of TGF-β1 and LAP is referred to as the small latent complex (SLC).
Fig. 1.
TGF-β1 latent complexes from Tgfb1+/+ and Tgfb1C33S/C33S mice. (A) Structure of latent TGF-β LLC. The LLC of TGF-β1 consists of the mature cytokine dimer, the propeptide dimer (LAP), and LTBP. The LAP residues that bind to LTBP are cysteines 33. Changing this residue to serine prevents formation of the TGF-β1 LLC. (B) LAP associations in Tgfb1+/+ and Tgfb1C33S/C33S lung cell culture media as revealed after SDS/PAGE and immunoblotting. Immunoreactive material from Tgfb1C33S/C33S cells is detected only at the position of the LAP dimer (75 kDa). (Left) There is no band at the position of LAP-LTBP. LAP immunoreactive material is present at the position corresponding to 250 kDa, the position of LAP plus LTBP. (Center) Western blot of Tgfb1C33S/C33S cell conditioned medium with an antibody to LTBP-3. (Right) The positive reaction indicates that the LLC is composed of LAP and LTBP-3. Blotting with antibodies to LTBP-1 revealed no LLC indicating the absence or undetectable levels of this species in either sample. Antibody to LTBP-4 was not tested.
Within the LLC, the functions of TGF-β and LAP are clear: TGF-β is the potential signaling molecule and LAP confers latency, thereby regulating the time and place of TGF-β action. The release of TGF-β from inhibition by LAP is referred to as latent TGF-β activation. Latent TGF-β can be activated by several different molecules, including proteases, thrombospondin-1, reactive oxygen species, and the integrins αvß6 and αvß8 (1).
The functions of LTBP, however, are not well understood (4, 5). LTBPs may act as intracellular chaperones for the SLC. In the absence of LTBP, SLC is secreted slowly and the liberated complex contains incorrectly paired disulfide bonds, whereas in the presence of LTBP, the secretion of TGF-β is enhanced and disulfide bond formation is correct (6). LTBPs may regulate latent TGF-β sequestration in the extracellular matrix (ECM) (4). Both immunochemical and biochemical approaches have revealed the presence of LLC in the ECM and association of the LTBP with fibronectin and fibrillin (4, 7, 8). Failure of LLC to associate properly with the matrix results in increased active TGF-β and abnormalities in skin and lung (9, 10). LTBP-1 participates in latent TGF-β activation by the integrin αvß6. αvß6 binds to Arg–Gly–Asp (RGD) sequences present in TGF-β1 and TGF-β3 LAPs and activates latent TGF-β via this interaction (11). However, a mutant of TGF-β1 in which cysteine 33, which normally forms the disulfide bonds with LTBP, is mutated to serine, thereby precluding LLC formation but permitting SLC formation, was not activated by αvβ6 (12). This result indicates a requirement for LTBP-1 in αvβ6-mediated latent TGF-β activation. LTBP-1 binds to the ECM protein fibronectin (13), enabling the cells to apply force to the latent complex via integrin binding to LAP, thereby releasing the TGF-β1 (14, 15).
Null or hypomorphic mutations in the Ltbp-1L, Ltbp-3, and Ltbp-4 genes have yielded mice with abnormalities in the development of the heart, bones, and lungs, respectively (16–19). The observed phenotypes all have been related to decreased TGF-β activity as described in other mouse models (20–23), further supporting the proposed importance of the LLC in TGF-β function.
These data indicate that LTBPs may assume multiple roles with regard to TGF-β action—as enhancers of SLC secretion, as SLC matrix localizers, and as participants in the activation of latent TGF-β. However, the interpretation of certain results can be criticized for several reasons including problems with overexpression, loss of TGF-β-independent functions, compensation by other LTBPs, and the difficulty of characterizing the effects of LTBP on latent TGF-β activation, if the absence of LTBP impedes SLC secretion.
To address the role of LTBP in TGF-β action and to avoid the caveats described above, we generated a mutant mouse in which the cysteine residue (Cys 33) that binds TGF-β1 LAP to LTBP was mutated to serine. In these Tgfb1C33S/C33S mice, TGF-β1 LAP cannot covalently complex with any LTBP, and all TGF-β1 is secreted as SLC. TGF-β1C33S generates a form of SLC that is effectively secreted (12). This ensures that the phenotypes of Tgfb1C33S/C33S mice represent a lack of LLC formation and not the loss of either SLC or an LTBP. We reasoned that if LLC formation is essential for TGF-β function, Tgfb1C33S/C33S mice should resemble Tgfb1−/− mice, which have multiorgan inflammation, die within 3 weeks of birth, and lack epidermal Langerhans cells (LC). Alternatively, if LLC formation is not critical for TGF-β function, Tgfb1C33S/C33S mice should resemble wild-type mice.
Results
Generation of Tgfb1C33S/C33S Mice.
Two lines of Tgfb1C33S/C33S mice were generated as described in Methods and supporting information (SI) Fig. S1. The Tgfb1C33S genotype is distinguished experimentally from the Tgfb1 wild-type allele by the loss of the BfuAI restriction site and/or by the presence of the lox sequence in the first intron of the Tgfb1 gene.
Wild-type (Tgfb1+/+), heterozygous (Tgfb1+/C33S), and Tgfb1C33S/C33S mice are born at essentially normal Mendelian ratios (Table S1). The two lines appeared equivalent in all of the analyses described below. Tgfb1C33S/C33S mice can survive for 5 months, although 50% of the animals die within 30 days (Fig. S2A). The fact that all Tgfb1C33S/C33S mice are born and many survive for several months is in contrast to Tgfb1−/− mice that either die in utero or within 3 weeks of birth (24). The cause of death in Tgfb1C33S/C33S mice is unknown, but at necropsy some of the Tgfb1C33S/C33S animals had thrombi in their right atria. Interestingly, a mutant form of LAP-β1 with Cys33Ser substitution overexpressed in mouse heart yields atrial fibrosis and delayed myocardial healing (25). We did not observe this in our mice, perhaps because we did not use overexpression of Tgfb1C33S/C33S. Tgfb1C33S/C33S animals appeared physically normal at birth, but by 4 weeks, differences in weight were apparent between homozygous mutant vs. Tgfb1+/C33S and Tgfb1+/+ mice (Fig. S2B). These differences persisted for the remainder of the life of the mice (Fig. S2B).
The prolonged survival of Tgfb1C33S/C33S mice compared to Tgfb1−/− mice suggested that association of LTBP with SLC was dispensable for TGF-β1 function. To ensure that Tgfb1C33S/C33S mice produced only SLC, we characterized TGF-β1 from cultures of immortalized cells derived from Tgfb1+/+ and Tgfb1C33S/C33S lungs (26). We verified the lack of TGF-β1 LAP association with LTBP by Western blot analysis of conditioned media from Tgfb1+/+ and mutant cell cultures after nonreducing SDS/PAGE (Fig. 1B). In the Tgfb1+/+ sample we detected a single 250-kDa band by both anti-LAP and anti-LTBP antibodies. This band corresponds to the covalent complex of LAP and LTBP, as SDS dissociates TGF-β1 from the LLC; therefore, no LLC or SLC is visible after SDS/PAGE. Immunoreactive protein from Tgfb1C33S/C33S cells was observed at the position corresponding to 75 kDa, the molecular mass of the TGF-β1 LAP dimer, but there was no immunoreactive material at the 250-kDa position. Thus, covalent complexes did not form between the mutant LAP and LTBP in Tgfb1C33S/C33S mice.
Langerhans Cells in Tgfb1C33S/C33S Mice.
The early death of Tgfb1C33S/C33S mice compared to Tgfb1+/+ or Tgfb1+/C33S animals was reminiscent of the early mortality of Tgfb1−/− mice. Therefore, we examined the Tgfb1C33S/C33S mice for additional phenotypes associated with TGF-β1 deficiency. A striking phenotype of Tgfb1−/− mice is the absence of LC in the epidermis (27). To test whether Tgfb1C33S/C33S mice contain LC in their epidermis, we stained sheets of back skin epidermis from 21-week-old Tgfb1+/+ and Tgfb1C33S/C33S mice for LC (28). No LC were detected in the epidermis of the mutant mice (Fig. S3), consistent with decreased active TGF-β1 generation in Tgfb1C33S/C33S mice.
Inflammation in Tgfb1C33S Mice.
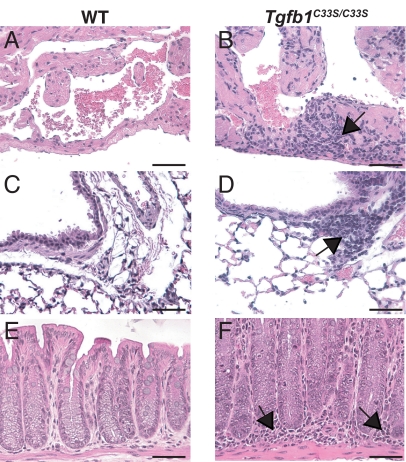
Another early phenotype in Tgfb1 null mice is multiorgan inflammation (29, 30). Therefore, we analyzed multiple tissues from Tgfb1+/+ and Tgfb1C33S/C33S mice of different ages for inflammation. Histological analysis of tissues of Tgfb1C33S/C33S mice revealed inflammation of the heart, lungs, and rectum (Fig. 2) and the stomach, colon, cecum, and anus (data not shown) by 10 weeks of age. There was no observable inflammation of the skin, liver, prostate, or pancreas (data not shown). In the GI tract, inflammation was observed as early as 5 weeks after birth. There was some variability in the degree of inflammation, and animals that survived for long periods (3–4 months) exhibited little to no inflammation. Within the lungs, inflammation was seen around large blood vessels and large airways (data not shown). In the rectum, inflammatory cells were observed in the lamina propria and surrounding the crypts. As TGF-β1 is a potent suppressor of inflammation (31), the inflammation in the Tgfb1C33S/C33S mice probably reflects decreased active TGF-β1 produced in the affected organs. However, the inflammation in Tgfb1C33S/C33S mice was not as severe as that seen in Tgfb1−/− mice (29, 30) suggesting that the Tgfb1C33S/C33S mice are hypomorphs, rather than nulls, for TGF-β1 formation.
Fig. 2.
Inflammation in Tgfb1+/+ and Tgfb1C33S/C33S tissues. Samples were taken from WT and Tgfb1C33S/C33S fixed, sectioned, and stained. (A, C, and E) Tgfb1+/+ tissues. (B, D, and E) Tgfb1C33S/C33S tissues. (A and B) Heart. There is no inflammation in the Tgfb1+/+ heart, but inflammatory cells (arrows) are present in the mutant heart. (C and D) Lung. Although the numbers of inflammatory cells in the Tgfb1+/+ lung are low, in the mutant tissue significant numbers of inflammatory cells surround certain blood vessels (arrows). (E and F) Terminal colon/rectum. The tissue from the Tgfb1+/+ mouse displays few inflammatory cells. However, the tissue from the Tgfb1C33S/C33S animal had large numbers of inflammatory cells in the lamina propria. The heart and lung tissues were from 4-week-old animals and the terminal colon/rectal tissue from 12-week-old mice. (Scale bar: 50 μM.)
Tumors in Tgfb1C33S/C33S Mice.
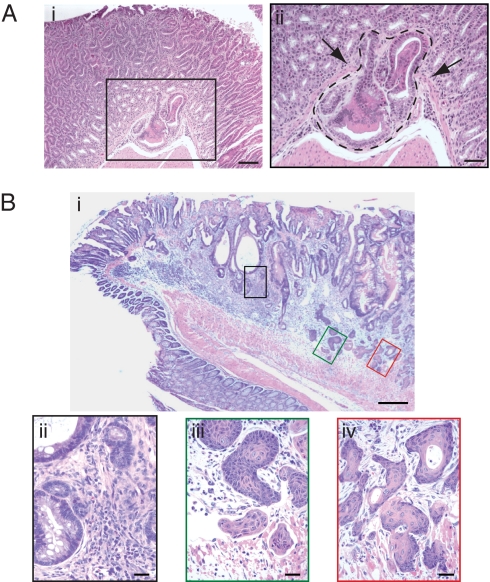
Histological examination of tissues from Tgfb1C33S/C33S mice revealed tumors in multiple organs (Table 1). Neoplasms were observed in ≈40% of the mutant animals 8 weeks of age (Table 1). We observed papillomas in the forestomach (data not shown) and adenocarcinomas in the glandular stomach (Fig. 3A), adenosquamous carcinomas in the terminal colon/rectum (Fig. 3B), and squamous cell carcinomas in the anus (data not shown). A few animals had tumors at multiple sites (Table 1). We found no tumors in the more proximal colon, skin, or lungs. The absence of tumors at more proximal sites in the colon of Tgfb1C33S/C33S mice may reflect a longer latency period for tumor development, as colon tumors in Tgfb1−/− mice develop significantly later than the tumors we observed. Tumor development correlated with the intensity of inflammation, as animals with mild inflammation rarely had tumors, whereas mice with strong inflammation almost always had tumors. The appearance of tumors in Tgfb1C33S/C33S mice is consistent with decreased levels of active TGF-β1 and a requirement for LLC formation for proper active TGF-β formation.
Table 1.
Incidence of neoplasms in Tgfb1C33S/C33S mice
| Tumors | Genotype |
||
|---|---|---|---|
| Tgfb1+/+ | Tgfb1+/C33S | Tgfb1C33S/C33S | |
| + | 0 | 0 | 21 |
| − | 29 | 24 | 30 |
Tumor frequency in Tgfb1+/+, Tgfb1+/C33S, and Tgfb1C33S/C33S mice. All mice were >8 weeks old when killed. There were 12 gastric papillomas, 4 gastric adenocarcinomas, 5 anal squamous cell carcinomas, 4 rectal adenocarcinomas, and 1 cecal adenocarcinoma. Four mice had both a squamous cell carcinoma and a rectal adenocarcinoma, two mice had a gastric adenocarcinoma and a rectal carcinoma, one mouse had a gastric papilloma and a cecal adenocarcinoma.
Fig. 3.
Tumors in Tgfb1C33S/C33S mice. (Ai) Section from the glandular stomach mucosa illustrating an early gastric adenocarcinoma. (ii) Higher magnification of the enclosed box in Ai. Arrows point to invasive dysplastic epithelial cells that have breached the basement membrane and muscularis mucosa. (B) (i) Section near the rectal–anal junction illustrating an adenosquamous carcinoma. (ii–iv) Higher magnification of regions comprising the boxed areas shown in B. (Biii) Tumor with adenomatous histology. (iii–iv) Tumor with squamous cell carcinoma histology. [Scale bars: 100 μM (Ai); 50 μM (Aii); 200 μM (Bi); 50 μM (Bii); and 20 μM (B iii and iv).]
TGF-β Signaling.
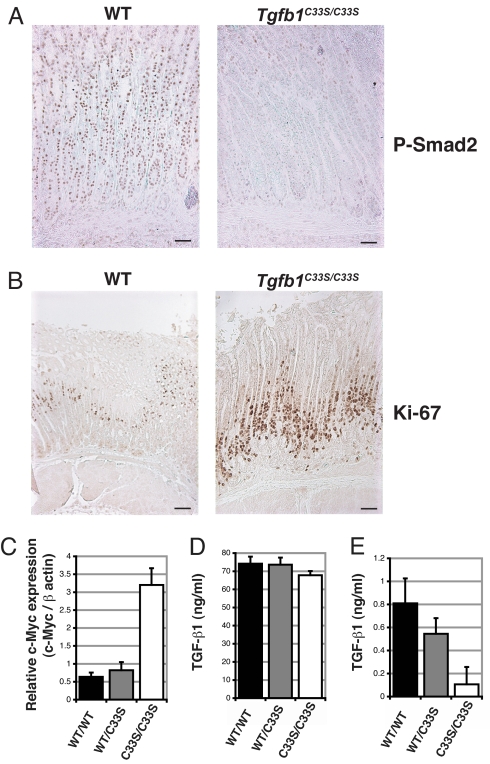
The phenotypes of Tgfb1C33S/C33S mice indicate decreased levels of TGF-β signaling. Therefore, we next examined the tissues of Tgfb1C33S/C33S mice for impaired TGF-β signaling activity. TGF-β1 signaling is propagated intracellularly by Smad2 and -3, which upon TGF-β receptor activation are phosphorylated, complex with Smad4, relocate in the nucleus, and form transcription complexes at TGF-β-responsive genes (2). Nuclear localization of P-Smad2 and -3, therefore, reflects TGF-β signaling within tissues. We examined the distribution of P-Smad2 in the stomachs of Tgfb1+/+ and Tgfb1C33S/C33S mice at 11 weeks in animals with little inflammation and found that the nuclear staining of the mutant cells was less abundant and less intense than that of Tgfb1+/+ cells, indicating decreased TGF-β signaling (Fig. 4A). We observed similar differences in the lungs at 8 weeks (data not shown). TGF-β acts as a suppressor of cell division in many epithelia. Therefore, we examined stomach and rectal epithelia of Tgfb1C33S/C33S mice for dividing cells with an antibody to Ki67 (32). We observed increased numbers of positively stained cells in the mutant compared to Tgfb1+/+ in both the stomach (Fig. 4B) and the rectal (data not shown) epithelium consistent with decreased levels of TGF-β in the mutant tissue. We measured Myc expression in mutant rectal tissue using Q-RT-PCR (Fig. 4C), as C-Myc expression is often suppressed by TGF-β (33). C-Myc levels were enhanced in the mutant vs. the Tgfb+/+ tissue. The expression of two other genes Pai-1 and Ctgf, which are regulated by TGF-β, was not altered in mutant compared to normal tissue (data not shown), perhaps because of opposing effects of the inflammatory response. Overall, these results indicate decreased active TGF-β1 generation in Tgfb1C33S/C33S mice.
Fig. 4.
TGF-β signaling and mitosis in tissues from Tgfb1+/+ and Tgfb1C33S/C33S mice. (A) P-Smad2 staining of stomach from Tgfb1+/+ and Tgfb1C33S/C33S of 11-week-old mice. The more intense P-Smad2 nuclear staining in the Tgfb1+/+ vs. the Tgfb1C33S/C33S sample indicates enhanced TGF-β activity. (B) Staining of stomach tissue from Tgfb1+/+ and Tgfb1C33S/C33S 21-week-old mice with antibody to Ki67, a marker of mitosis. The number of positive Ki67 cells is higher in the mutant sample compared to the Tgfb1+/+ sample, consistent with decreased TGF-β1 in the tissue, as TGF-β1 is a suppressor of growth of most epithelial cells. (C) Q-RT-PCR of stomach epithelium for Myc from 12-week-old mice. Tgfb1C33S/C33S tissue had higher expression of Myc than did control tissue consistent with decreased TGF-β1 in the mutant tissue. (D and E) TGF-β1 in sera from Tgfb1+/+ and Tgfb1C33S/C33S mice. TGF-β1 in sera from Tgfb1+/+, Tgfb1+/C33S, and Tgfb1C33S/C33S mice was measured by an ELISA specific for active TGF-β1. (D) Total TGF-β1 in sera after acidification. Acidification releases all active TGF-β from its latent complex. The total TGF-β1 from the three genotypes was equivalent. (E) Active TGF-β1 in sera. TGF-β1 was measured directly in sera without acidification. The amount of active TGF-β1 in the Tgfb1+/+ and Tgfb1+/C33S samples is significantly higher than that found in samples from Tgfb1C33S/C33S mice. Samples from 10–12 animals of each genotype were assayed for TGF-β. Assays on each serum sample were done in triplicate. Values are presented as SEM.
Decreased TGF-β1 signaling in Tgfb1C33S/C33S mice might result from decreased synthesis, processing, or secretion of TGF-β1C33S. Therefore, we assayed sera from Tgfb1+/+ and Tgfb1C33S/C33S mice for TGF-β1 by ELISA to assess total and active TGF-β1 levels in vivo. When we measured the total amount of TGF-β1 in each sample after acid treatment, a process that releases all latent TGF-β from LLC and SLC, we found only a slight difference between Tgfb1+/+ and Tgfb1C33S/C33S samples, indicating no significant inhibition of synthesis, processing, or release of the mutant protein (Fig. 4D). Untreated sera from either Tgfb1+/+ or Tgfb1C33S/C33S mice contained <1% active TGF-β1 compared to the acid-treated samples. But the Tgfb1+/+ and Tgfb1+/C33S samples contained significantly more active TGF-β1 than did the Tgfb1C33S/C33S samples (Fig. 4E). Therefore, we conclude that the mutant mice produce approximately normal levels of latent TGF-β1, but the lack of LLC formation impairs latent TGF-β1 activation.
Discussion
We generated Tgfb1C33S/C33S mice to test for a requirement for LLC formation in TGF-β action. The absence of the cysteine 33 in TGF-β1 LAP precluded covalent association of any LTBP with the TGF-β1 SLC yielding exclusively the TGF-β1 SLC. The inability of the mutant SLC to bind to LTBP was demonstrated in conditioned medium from cultured cells derived from the lungs of Tgfb1C33S/C33S mice (Fig. 1B). Analysis of active TGF-β1 levels in serum from control and Tgfb1C33S/C33S animals indicated that mutant sera contained less active TGF-β1 than did Tgfb1+/+ sera supporting a role for LLC formation in latent TGF-β1 activation. Although noncovalent interactions can occur between LAP and LTBP (34), we think that this type of association is unlikely to result in biologically significant extracellular interaction, as Tgfb1C33S/C33S has been shown to yield defective activation in two systems (12, 35). Therefore, we hypothesize that the hypomorphic, rather than null, phenotype of this mutation reflects activation of the SLC by activators such as TSP-1 (36) or αvβ8 (37).
Tgfb1C33S/C33S mice display a phenotype consistent with systemic decreased levels of active TGF-β1 compared to Tgfb1+/+ animals. Like Tgfb1−/− mice, Tgfb1C33S/C33S mice have multiorgan inflammation and lack LC in the epidermis. Tgfb1C33S/C33S mice also display decreased levels of nuclear P-Smad2 in the stomach and lungs, and increased C-Myc expression and increased numbers of mitotic cells, all properties associated with decreased TGF-β. However, Tgfb1C33S/C33S mice have a milder multiorgan inflammation than Tgfb1−/− mice and have a longer life span. Tgfb1C33S/C33S animals develop neoplasms of the GI tract without suppression of the immune response, a phenotype absent in Tgfb1−/− mice (38). Tumor occurrence in Tgfb1C33S/C33S, but not in Tgfb1−/−, mice may relate to the longer survival of the Tgfb1C33S/C33S animals, as Tgfb1−/− mice develop tumors when their life span is extended (38). However, the tumor spectrum in Tgfb1C33S/C33S mice is different from that reported in immunosuppressed Tgfb1−/− mice, which develop colon carcinomas (38). Therefore, although Tgfb1C33S/C33S mice resemble Tgfb1−/− mice, they are not phenocopies. Rather Tgfb1C33S/C33S mice appear to be hypomorphic, not null, for active TGF-β1.
The phenotype of the Tgfb1C33S/C33S mice should be compared to Tgfb1RGE/RGE mice, which produce TGF-β1 that has an Arg–Gly–Glu (RGE) rather than RGD sequence in LAP (39). Absence of the RGD sequence precludes binding of either of the two integrins, αvß6 and αvß8, known to activate latent TGF-β1 (11, 37), to the TGF-β1 LAP. Tgfb1RGE/RGE mice are phenocopies of Tgfb1−/− mice, with strong multiorgan inflammation, lack of epidermal LC, and early death (39). These results indicate that the early Tgfb1−/− phenotypes derive from the absence of TGF-β1 generated by the action of αvβ6 and αvβ8 integrins. We reported that activation of latent TGF-β1 by αvβ6 required the participation of LTBP-1, and that TGF-β1C33S/C33S SLC was not activated by this integrin (12). We hypothesize that the lack of LC and the multiorgan inflammation observed in Tgfb1C33S/C33S mice result from decreased SLC compared to LLC activation. The fact that Tgfb1C33S/C33S mice are not identical to Tgfb1−/− or Tgfb1RGE/RGE mice may relate to the ability of the integrin αvβ8 to activate SLC (37). It will be interesting to determine the consequence of blocking αvβ8-mediated activation of latent TGF-β in Tgfb1C33S/C33S mice.
Finally, it is noteworthy that Tgfb1C33S/C33S mice develop tumors in multiple locations in the GI tract, a particularly sensitive region for tumor induction upon decreased TGF-β signaling. Colon tumors occur in Tgfb1−/− mice, if the inflammatory response is eliminated by crossing the null mutation onto a Rag2−/− background (38). Colon tumors also occur in other mouse models, such as Smad3−/− and ApcΔ716 Smad4+/− mice, in which TGF-β signaling is impaired (40, 41). In addition, human colon carcinomas are often associated with mutations in components of the TGF-β signaling pathway (42–44). Previously, it was assumed that TGF-β acted directly on the epithelial cells and the loss of TGF-β signaling permitted initiated cells to proliferate. Recently, mutant mice were described in which TGF-β signaling was suppressed either in stromal cells or in T cells, but the mice develop tumors of the epithelia of the stomach or the intestine (45, 46), i.e., in tissues that have normal TGF-β signaling. Therefore, the effects of TGF-β in the tumor microenvironment may be critical in controlling tumor growth. We do not know whether tumors in the GI tract of Tgfb1C33S/C33S mice result from decreased TGF-β1 produced by the epithelial cells, the surrounding cells, or both. This will be an interesting question to answer in future experiments.
Materials and Methods
Plasmids.
The pKS10xPNT plasmid was a gift of A. Joyner (Memorial Sloan-Kettering Institute).
Construction of the Tgfb1 targeting vector. See SI Methods.
Generation of Tgfb1C33S/C33S mice. See SI Methods.
Primary Fibroblasts.
Primary fibroblasts were obtained from the minced lungs of 6-day-old genotyped mice. Cells were immortalized by Sv40 large T as described (26).
TGF-β Assays.
TGF-β1 concentrations were determined using the TGF-β1 Quantikine kit (R&D Systems). For detect active TGF-β1 in serum, samples were assayed without acid treatment. To detect total TGF-β1, latent TGF-β1 in serum was activated by acid treatment according to the manufacturer's instructions. All samples were quantified using the linear portion of a standard curve generated with the same kit and recombinant TGF-β1 (R&D Systems). Sera were prepared from anesthetized mice by heart puncture with a 27-g needle and a 1-ml syringe. Blood was immediately placed in a glass tube, allowed to clot at 37 °C for 4 h, and the sera obtained after centrifugation at 4 °C for 20 min at 14,000 g.
Immunohistochemistry.
Three-micrometer sections of fresh formalin-fixed tissues were used. Unless otherwise noted, all staining was with hematoxylin and eosin. Endogenous peroxidase activity was quenched by incubation with 3% hydrogen peroxide in 100% methanol for 30 min. Antigen retrieval was performed using a Dako Cytomation Target Retrieval Solution for 20 min at 95 °C or in Na-citrate (0.01 M, pH 6), heated for 10 min in a microwave oven. Primary antibodies used were: phospho-Smad2 (1:300 dilution; Chemicon), Ki67 (1:400; Novocastra), and I-A/I-E (Clone 269; Pharmagen). Secondary staining used biotinylated secondary antibodies (Vector Laboratories) followed by Elite Vectastain ABC kit (Vector Laboratories) and peroxidase substrate DAB kit (Vector Laboratories).
LC Immunostaining.
Preparation of dorsal trunk epidermal sheets and staining was done as described (27, 28).
Quantitative Real-Time RT-PCR Analysis.
RNA was extracted from 10 pairs of Tgfb1+/+ and Tgfb1C33S/C33S rectums using TRIzol (Invitrogen). Reverse transcription (RT) was performed using 1 μg of RNA and the SuperScript III Reverse Transcriptase (Invitrogen) (50 °C, 60′). The resulting cDNA was used for quantitative real-time RT-PCR (Q-RT-PCR) analysis (47). Q-RT-PCRs were performed using specific primers and QuantiFast SYBR Green PCR Kit (Qiagen) on an iCycler Thermal Cycler (Bio-Rad). Each target transcript expression was quantified by comparing the threshold cycle (TC) with that of hypoxanthine guanine phosphoribosyl transferase by using the comparative TC method (19). Primers used: Actb sense AGC CTT CCT TCT TGG GTA TGG, antisense GCC ACC GAT CCA CAC AGA GTA; Myc sense GCT GCT GTC CTC CGA GTC CTC, antisense GGG GTT TGC CTC TTC TCC ACA.
Statistical Analyses.
Descriptive statistics were performed with StatView J-4.5 program (SAS Institute). The Kaplan–Meier method was used to estimate all survival curves from mouse studies. The log-rank statistic was used to compare the overall survival distributions of Tgfb1+/+ and Tgfb1C33S/C33S mice. Mouse survival curves were compared with the log-rank statistic.
Supplementary Material
Acknowledgments.
This work was supported by National Institutes of Health grants CA034282 (D.B.R.), F32 HL67542–01 (V.J.), T32 CA09161 (V.J.), AR 049698 (D.B.R. and D.L.H.), and Phillip Morris Foundation (D.B.R.). K.Y. was supported by a fellowship from the Uehara Foundation. H.O. was supported by a Bausch and Lomb Overseas Research Fellowship.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805411105/DCSupplemental.
References
- 1.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFbeta activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 2.Derynck R, Miyazono K. The TGF-â Family. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saharinen J, Keski-Oja J. Specific sequence motif of 8-Cys repeats of TGF-beta binding proteins, LTBPs, creates a hydrophobic interaction surface for binding of small latent TGF-beta. Mol Biol Cell. 2000;11:2691–2704. doi: 10.1091/mbc.11.8.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: Orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–7412. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 5.Todorovic V, et al. Latent TGF-beta binding proteins. Int J Biochem Cell Biol. 2005;37:38–41. doi: 10.1016/j.biocel.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 6.Miyazono K, Olofsson A, Colosetti P, Heldin CH. A role of the latent TGF-beta 1-binding protein in the assembly and secretion of TGF-beta 1. EMBO J. 1991;10:1091–1101. doi: 10.1002/j.1460-2075.1991.tb08049.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dallas SL, et al. Fibronectin regulates latent transforming growth factor-beta (TGF beta) by controlling matrix assembly of latent TGF beta-binding protein-1. J Biol Chem. 2005;280:18871–18880. doi: 10.1074/jbc.M410762200. [DOI] [PubMed] [Google Scholar]
- 8.Isogai Z, et al. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem. 2003;278:2750–2757. doi: 10.1074/jbc.M209256200. [DOI] [PubMed] [Google Scholar]
- 9.Mazzieri R, et al. Expression of truncated latent TGF-beta-binding protein modulates TGF-beta signaling. J Cell Sci. 2005;118:2177–2187. doi: 10.1242/jcs.02352. [DOI] [PubMed] [Google Scholar]
- 10.Neptune ER, et al. Dysregulation of TGF-beta activation contributes to pathogenesis in Marfan syndrome. Nat Genet. 2003;33:407–411. doi: 10.1038/ng1116. [DOI] [PubMed] [Google Scholar]
- 11.Munger JS, et al. The integrin αvβ6 binds and activates latent TGFβ1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1998;96:319–328. doi: 10.1016/s0092-8674(00)80545-0. [DOI] [PubMed] [Google Scholar]
- 12.Annes JP, Chen Y, Munger JS, Rifkin DB. Integrin alphaVbeta6-mediated activation of latent TGF-beta requires the latent TGF-beta binding protein-1. J Cell Biol. 2004;165:723–734. doi: 10.1083/jcb.200312172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fontana L, et al. Fibronectin is required for integrin alphavbeta6-mediated activation of latent TGF-beta complexes containing LTBP-1. FASEB J. 2005;19:1798–1808. doi: 10.1096/fj.05-4134com. [DOI] [PubMed] [Google Scholar]
- 14.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent TGF- 1 from the extracellular matrix. J Cell Biol. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins RG, et al. Ligation of protease-activated receptor 1 enhances alpha (v) beta6 integrin-dependent TGF-beta activation and promotes acute lung injury. J Clin Invest. 2006;116:1606–1614. doi: 10.1172/JCI27183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dabovic B, et al. Bone abnormalities in latent TGF-β binding protein (Ltbp)-3-null mice indicate a role for Ltbp-3 in modulating TGF-β bioavailability. J Cell Biol. 2002;156:227–232. doi: 10.1083/jcb.200111080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dabovic B, et al. Bone defects in latent TGF-beta binding protein (Ltbp)-3 null mice; a role for Ltbp in TGF-beta presentation. J Endocrinol. 2002;175:129–141. doi: 10.1677/joe.0.1750129. [DOI] [PubMed] [Google Scholar]
- 18.Sterner-Kock A, et al. Disruption of the gene encoding the latent transforming growth factor-beta binding protein 4 (LTBP-4) causes abnormal lung development, cardiomyopathy, and colorectal cancer. Genes Dev. 2002;16:2264–2273. doi: 10.1101/gad.229102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Todorovic V, et al. Long form of latent TGF-{beta} binding protein 1 (Ltbp1L) is essential for cardiac outflow tract septation and remodeling. Development. 2007;134:3723–3732. doi: 10.1242/dev.008599. [DOI] [PubMed] [Google Scholar]
- 20.Choudhary B, et al. Cardiovascular malformations with normal smooth muscle differentiation in neural crest-specific type II TGFbeta receptor (Tgfbr2) mutant mice. Dev Biol. 2006;289:420–429. doi: 10.1016/j.ydbio.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Erlebacher A, Derynck R. Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype. J Cell Biol. 1996;132:195–210. doi: 10.1083/jcb.132.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markowitz S, et al. Inactivation of the type II TGF-beta receptor in colon cancer cells with microsatellite instability. Science. 1995;268:1336–1338. doi: 10.1126/science.7761852. [DOI] [PubMed] [Google Scholar]
- 23.Riggins GJ, Kinzler KW, Vogelstein B, Thiagalingam S. Frequency of Smad gene mutations in human cancers. Cancer Res. 1997;57:2578–2580. [PubMed] [Google Scholar]
- 24.Kallapur S, Ormsby I, Doetschman T. Strain dependency of TGFbeta1 function during embryogenesis. Mol Reprod Dev. 1999;52:341–349. doi: 10.1002/(SICI)1098-2795(199904)52:4<341::AID-MRD2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 25.Nakajima H, et al. Atrial but not ventricular fibrosis in mice expressing a mutant transforming growth factor-beta(1) transgene in the heart. Circ Res. 2000;86:571–579. doi: 10.1161/01.res.86.5.571. [DOI] [PubMed] [Google Scholar]
- 26.Peterson SR, Gadbois DM, Bradbury EM, Kraemer PM. Immortalization of human fibroblasts by SV40 large T antigen results in the reduction of cyclin D1 expression and subunit association with proliferating cell nuclear antigen and Waf1. Cancer Res. 1995;55:4651–4657. [PubMed] [Google Scholar]
- 27.Borkowski TA, Letterio JJ, Farr AG, Udey MC. A role for endogenous transforming growth factor beta 1 in Langerhans cell biology: The skin of transforming growth factor beta 1 null mice is devoid of epidermal Langerhans cells. J Exp Med. 1996;184:2417–2422. doi: 10.1084/jem.184.6.2417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas RM, et al. Appearance of Langerhans cells in the epidermis of Tgfb1(−/−) SCID mice: Paracrine and autocrine effects of transforming growth factor-beta 1 and -beta 2(1) J Invest Dermatol. 2001;117:1574–1580. doi: 10.1046/j.0022-202x.2001.01550.x. [DOI] [PubMed] [Google Scholar]
- 29.Kulkarni AB, et al. Transforming growth factor ß1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci USA. 1993;90:770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-ß1 gene results in mulitfocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Visser KE, Kast WM. Effects of TGF-beta on the immune system: Implications for cancer immunotherapy. Leukemia. 1999;13:1188–1199. doi: 10.1038/sj.leu.2401477. [DOI] [PubMed] [Google Scholar]
- 32.Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- 33.Warner BJ, Blain SW, Seoane J, Massague J. Myc downregulation by transforming growth factor beta required for activation of the p15(Ink4b) G(1) arrest pathway. Mol Cell Biol. 1999;19:5913–5922. doi: 10.1128/mcb.19.9.5913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, et al. Amino acid requirements for formation of the TGF-beta-latent TGF-beta binding protein complexes. J Mol Biol. 2005;345:175–186. doi: 10.1016/j.jmb.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 35.Ahamed J, et al. In vitro and in vivo evidence for shear-induced activation of latent transforming growth factor-{beta}1 (TGF-{beta}1) Blood. 2008;112:3650–3660. doi: 10.1182/blood-2008-04-151753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schultz-Cherry S, Ribeiro S, Gentry L, Murphy-Ullrich E. Thrombospondin binds and activates the small and large forms of latent transforming growth factor-ß in a chemically defined system. J Biol Chem. 1994;269:26775–26782. [PubMed] [Google Scholar]
- 37.Mu D, et al. The integrin alpha (v) beta8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-beta1. J Cell Biol. 2002;157:493–507. doi: 10.1083/jcb.200109100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Engle SJ, et al. Transforming growth factor beta1 suppresses nonmetastatic colon cancer at an early stage of tumorigenesis. Cancer Res. 1999;59:3379–3386. [PubMed] [Google Scholar]
- 39.Yang Z, et al. Absence of integrin-mediated TGFbeta1 activation in vivo recapitulates the phenotype of TGFbeta1-null mice. J Cell Biol. 2007;176:787–793. doi: 10.1083/jcb.200611044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takaku K, et al. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 41.Zhu Y, Richardson JA, Parada LF, Graff JM. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- 42.Eppert K, et al. MADR2 maps to 18q21 and encodes a TGFbeta-regulated MAD-related protein that is functionally mutated in colorectal carcinoma. Cell. 1996;86:543–552. doi: 10.1016/s0092-8674(00)80128-2. [DOI] [PubMed] [Google Scholar]
- 43.Grady WM, et al. Mutational inactivation of transforming growth factor beta receptor type II in microsatellite stable colon cancers. Cancer Res. 1999;59:320–324. [PubMed] [Google Scholar]
- 44.Hahn SA, et al. DPC4, a candidate tumor suppressor gene at human chromosome 18q21.1. Science. 1996;271:350–353. doi: 10.1126/science.271.5247.350. [DOI] [PubMed] [Google Scholar]
- 45.Bhowmick NA, et al. TGF-beta signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 46.Kim BG, et al. Smad4 signalling in T cells is required for suppression of gastrointestinal cancer. Nature. 2006;441:1015–1019. doi: 10.1038/nature04846. [DOI] [PubMed] [Google Scholar]
- 47.Wang Y, Zhu W, Levy DE. Nuclear and cytoplasmic mRNA quantification by SYBR green based real-time RT-PCR. Methods. 2006;39:356–362. doi: 10.1016/j.ymeth.2006.06.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.