Abstract
The recent development of small-molecule tyrosine kinase (TK) inhibitors offers increasing opportunities for the treatment of autoimmune diseases. In this study, we investigated the potential of this new class of drugs to treat and cure type 1 diabetes (T1D) in the NOD mouse. Treatment of prediabetic and new onset diabetic mice with imatinib (Gleevec) prevented and reversed T1D. Similar results were observed with sunitinib (Sutent), an additional approved multikinase inhibitor, suggesting that the primary target of imatinib, c-Abl, was not essential in blocking disease in this model. Additional studies with another TK inhibitor, PLX647 (targeting c-Kit and c-Fms) or an anti-c-Kit mAb showed only marginal efficacy whereas a soluble form of platelet-derived growth factor receptor (PDGFR), PDGFRβIg, rapidly reversed diabetes. These findings strongly suggest that inhibition of PDGFR is critical to reverse diabetes and highlight a crucial role of inflammation in the development of T1D. These conclusions were supported by the finding that the adaptive immune system was not significantly affected by imatinib treatment. Finally, and most significantly, imatinib treatment led to durable remission after discontinuation of therapy at 10 weeks in a majority of mice. Thus, long-term efficacy and tolerance is likely to depend on inhibiting a combination of tyrosine kinases supporting the use of selective kinase inhibitors as a new, potentially very attractive approach for the treatment of T1D.
Keywords: autoimmunity, diabetes, tyrosine kinase inhibitor, imatinib, PDGFR
Imatinib (Gleevec) is a 2-phenylaminopyrimidine derivate designed as a specific inhibitor of the inactive conformation of Abl protein tyrosine kinases (1). Imatinib's activity against cells bearing the Bcr-Abl translocation has yielded remarkable results in the treatments of chronic myeloid leukemia (CML) with minimal side effects (2). In addition, imatinib has been found to be active against other tyrosine kinases such as platelet-derived growth factor receptor (PDGFR), c-Kit (CD117), macrophage colony stimulating factor receptor (c-Fms) and, perhaps, Lck (3–6). The inhibitory activity against c-Kit and PDGFR has enabled the development of effective treatments for gastrointestinal stromal tumors (7), eosinophilic disorders, and systemic mast cell disease (8, 9).
In addition to its direct effect on malignant cells, it has been suggested that imatinib treatment can have immunomodulatory activity. Studies have shown that imatinib treatment can result in impaired adaptive and innate immunity including T cell, dendritic cell (DC), and monocytes/macrophages function (10). Notably, several investigators have demonstrated antiinflammatory effects in various mouse models. Wolf et al. showed in a mouse model of acute hepatic inflammation that imatinib exhibited a strong antiinflammatory role by inhibiting TNF-α production in macrophages (11). Dietz et al. demonstrated that delayed-type hypersensitivity was reduced in mice treated with imatinib (12). Finally, imatinib has been shown to be effective in a number of rodent studies of autoimmunity. Imatinib efficiently prevented disease and induced remission in an autoimmune arthritis model (13, 14) and ameliorated autoimmune nephritis in a mouse model of lupus (15). These data are consistent with case reports and phase I studies in humans demonstrating a positive effect of imatinib on rheumatoid arthritis (16, 17), psoriasis (18), spondyloarthritis (19), and Crohn's disease (20).
Type 1 diabetes (T1D) is an autoimmune disease dependent on T cell-mediated destruction of insulin-producing β cells. Disease progression is strongly dependent on T cells, B cells, macrophages, and DCs. Importantly, recent studies have emphasized a role of inflammatory processes in β cell destruction and insulin resistance. To date there is no good immunotherapy to treat or prevent the development of this disease. T1D is characterized by the development of autoreactive antibodies and destructive T cell infiltration of insulin-producing islet β cells. The NOD (nonobese diabetes) mouse is an important model of autoimmune diabetes. Disease occurs spontaneously and shares many phenotypic and genetic similarities with T1D in human subjects (21). Lymphocyte infiltration of the islets of Langerhans begins at 2–4 weeks of age, progressing from periinsulitis to severe insulitis by 10 weeks of age. Diabetes onset typically occurs at 12–14 weeks in most female NOD mice. Given the overlap between the multiple targets of imatinib, previous results in other models of autoimmunity and the pathogenesis of autoimmune diabetes, we set out to test the hypothesis that this drug might be effective in preventing or treating this autoimmune disease. We show here that imatinib treatment can prevent and even reverse diabetes when administered to NOD mice. Furthermore, imatinib can be administered for as short as 10 weeks with long-lasting effects working through the inhibition of PDGFR. These results, coupled with recent studies demonstrating a direct protective effect of imatinib on type 2 diabetes in rodents (22) suggests that this molecule and other kinase inhibitors such as sunitinib have potential as a therapeutic to treat patients with this disease.
Results
Imatinib Prevents Development of Autoimmune Diabetes.
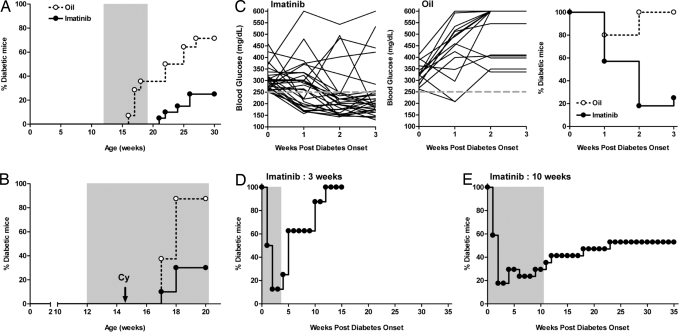
To test whether imatinib could alter diabetes development, we treated prediabetic NOD mice and followed incidence of diabetes. Commercially available Gleevec tablets were ground and suspended in peanut oil and given orally once a day at a dose of 1.5 mg/mouse. Treatment was initiated at 12 weeks, a prediabetic stage when a high degree of insulitis is already evident. During the 7 weeks of treatment, none of the imatinib-treated mice developed diabetes. By comparison, by 19 weeks, ≈40% of the oil-treated mice had developed disease (Fig. 1A). After cessation of treatment, 20% of the animals treated with imatinib became progressively diabetic by 30 weeks of age, as opposed to 71% of the oil-treated mice. The majority of imatinib-treated mice remained nondiabetic at >50 weeks of age, suggesting that the short-term therapy had long-term effects on the development of diabetes in this setting. We then assessed the effect of imatinib on cyclophosphamide (Cy)-induced diabetes. Cy-induced diabetes is a rapid onset robust model of disease, which has been linked to increased islet cell apoptosis and perhaps altered immune regulation (21). In this setting, only 30% of the 15-week-old animals treated with imatinib developed diabetes within 3 weeks after Cy injection (Fig. 1B). This result contrasted with the control oil-treated group of mice where fully 85% of the animals became diabetic during the same period. These results show that imatinib can prevent T1D in NOD mice.
Fig. 1.
Imatinib prevents and reverses development of autoimmune diabetes. (A) Prediabetic NOD mice (12 weeks of age) were treated (gavage) daily with imatinib (n = 20) or oil (n = 14) for 7 weeks (gray shaded area). Diabetes incidence is shown. Mice were determined diabetic with two consecutive readings of blood glucose >250 mg/dl. (B) Prediabetic female NOD mice were treated daily with imatinib (n = 10) or oil (n = 8). Cyclophosphamide (Cy, 300 mg/kg) was injected i.p., 2.5 weeks after beginning of treatment. Diabetes incidence is shown. (C) Imatinib or oil treatment of NOD mice was initiated at the time of disease onset (blood glucose >250 mg/dl) and continued for 3 weeks. Individual glucose readings and percentages of diabetic mice for imatinib-treated (n = 28) and oil-treated (n = 15) mice are shown. (D and E) Imatinib treatment (gray shaded area) was initiated at the time of disease onset and discontinued after 3 weeks (n = 8) or 10 weeks (n = 17). Percentages of diabetic mice are shown.
Imatinib Induces Remission of Established Diabetes.
Multiple approaches have been used to prevent spontaneous diabetes in the NOD mouse. However, there has been substantially less success in curing overtly diabetic NOD mice (23). Therefore, we tested whether imatinib could reverse diabetes when given at the time of new onset. Treatment with imatinib led to remission in 80% of new-onset diabetic mice. One week after initiation of treatment, imatinib reversed diabetes in >40% of the mice (Fig. 1C) but by 2 weeks, remission was observed in ≈80% of the mice (n = 28). Oil treatment did not reverse diabetes as all of the mice were diabetic (n = 15) 2 weeks after the beginning of the treatment (Fig. 1C). The imatinib effect was most notable in mice with blood glucose levels between 250 and 350 mg/dl at diagnosis but also in mice exhibiting higher glycemia (>300–450 mg/dl) although somewhat less effective. This difference most likely reflects the fact that efficacy of the imatinib may require several days of treatment, thus favoring remission in mice with substantial islet reserve at the time of the initiation of therapy.
Next, we examined whether imatinib treatment could be discontinued and maintain its efficacy. After cessation of therapy at 3 weeks, all mice became hyperglycemic by 10 weeks postdiagnosis (Fig. 1D). However, increasing the duration of treatment from 3 weeks to 10 weeks led to long-lasting diabetes remission. In the experiment depicted in Fig. 1E, a majority of the mice remained normal glycemic after cessation of therapy 10 weeks postdisease onset without the need of additional treatment.
Imatinib Does Not Significantly Diminish Leukocyte Accumulation Within the Pancreas.
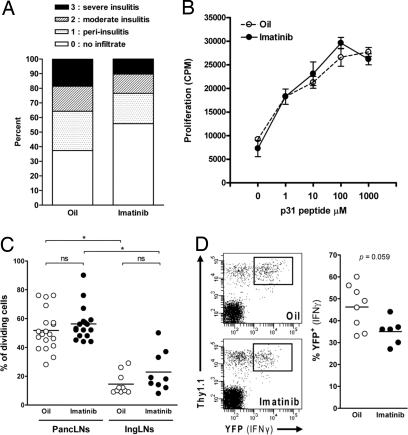
Prediabetic NOD mice were treated with imatinib or oil. After 2 weeks of treatment, mice were examined for the presence of mononuclear cell infiltrates. Although severe and mild insulitis were generally diminished in imatinib-treated mice compared with oil-treated mice (Fig. 2A), these differences were not statistically significant. Furthermore, longer treatment of 5 weeks did not reveal significant differences (data not shown). Finally, pancreatic islets from long-term normoglycemic mice (10-week treatment, Fig. 1E) were also examined. Although these mice were not diabetic, significant insulitis was still observed (data not shown). Thus, while preventing clinical disease, imatinib diminishes to some extent, but does not eliminate leukocyte infiltration of the pancreas.
Fig. 2.
Effect of imatinib on insulitis and T cells in vivo. (A) Prediabetic NOD mice (10 weeks of age) were treated daily with imatinib or oil for 2 weeks. Quantification of >280 pancreatic islets as a percentage for each histological score is shown (n = 8 mice/group). (B) Prediabetic NOD mice were treated with imatinib or oil for 5 weeks and spleen cell proliferation to p31 peptide (1–1000 μM) or anti-CD3 (0.1 μg/μL) was examined in vitro. Representative response from one mouse of each group is shown. (C) Prediabetic NOD mice were pretreated daily with imatinib or oil for 1 week before adoptive transfer of CD25-depleted CFSE-labeled BDC2.5-Thy1.1 congenic spleen and lymph node cells. Treatment was continued for 4 days and pancreatic (PancLNs) and inguinal (IngLNs) lymph nodes were subsequently collected to measure CFSE dilution (n = 9–19 mice/group, from five independent experiments). *P < 0.05. (D) Prediabetic NOD mice were pretreated with imatinib or oil for 1 week before adoptive transfer of CD4+CD25−YFP− T cells from BDC2.5.Thy1.1.Yeti mice. Treatment was continued for 1 week. Single-cell suspensions from isolated pancreatic islets were subsequently prepared to measure induction of YFP expression (reporter for IFN-γ expression). Gated on CD4+ cells.
Effect of Imatinib on T Cells in Vivo.
Both CD4+ and CD8+ T cells play an important role in the development of diabetes (21). Although several studies reported that imatinib could alter T cell function (12, 24), most of these effects were described when imatinib was added in vitro and using a relatively high concentration (13). Treatment of prediabetic NOD mice with the therapeutic dose of imatinib for 5 weeks did not change the CD4+/CD8+ T cell ratio in the spleen or pancreatic lymph nodes, nor did the therapy alter the absolute cell numbers of each subset when compared with oil-treated mice (data not shown). Similarly, the percentage of Foxp3+ among CD4+ T cells was not modified (data not shown). CD8+ T cells specific for islet-specific glucose-6-phosphatase catalytic subunit-related protein (25) were detectable in the islets of both oil- and imatinib-treated mice (supporting information (SI) Fig. S1). Antigen-specific CD4+ T cell proliferation was examined using the p31 peptide (1040-p31), a mimetope for an unknown islet antigen recognized by the diabetogenic BDC2.5 T cell (26). Spleen cells from imatinib- and oil-treated mice proliferated similarly when stimulated in vitro with p31 peptide (Fig. 2B). Furthermore, in vivo proliferation of the islet antigen-specific TCR transgenic mouse BDC2.5 was not affected in imatinib-treated mice compared with oil-treated mice (Fig. 2C). Finally, to determine whether imatinib could affect IFN-γ expression in T cells, we took advantage of the IFN-γ reporter mice known as “Yeti mice” (27). As shown in Fig. 2D, YFP expression was induced in ≈45% of the BDC2.5 Thy1.1+CD4+ T cells that infiltrated the islets in oil-treated mice. When recipient mice were treated with imatinib, the percentage of YFP+ cells decreased to ≈35%, although this difference did not reach significance (P = 0.059) when compared with oil-treated mice. Thus, at the dose that enables remission in new onset diabetic mice, imatinib marginally affects pathogenic T cell function, suggesting that imatinib does not achieve its effect on T1D through potent inhibition of Abl or Lck expressed in T cells.
Discrimination of Key Tyrosine Kinase Targets in the Diabetes Remission Induced by Imatinib.
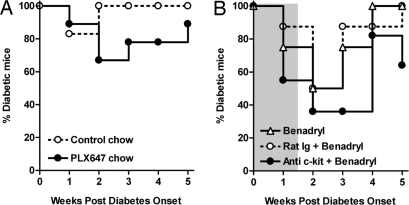
To identify which tyrosine kinase pathway(s), inhibited by imatinib, are key in the reversal of diabetes, we tested different TK blocking agents that share targets with imatinib (Table S1). First, we tested a small molecule, PLX647, which has been developed to inhibit c-Fms and c-Kit, specifically. As seen in Table S2, the IC50 for PLX647 suggests strong activity against KIT and CSF1R but not PDGFR-β when compared to imatinib and sunitinib allowing for separation of these kinase activities. Although there was a small effect of the ability of this drug to reverse diabetes after onset (Fig. 3A), the therapy was not nearly as efficacious as imatinib and not nearly as durable with the majority of mice relapsing while undergoing treatment. Next, we tested a mAb directed at the c-Kit cell surface molecule, ACK2. This mAb has previously been shown to block c-Kit and eliminate c-Kit+ cells in vivo (28). Unfortunately, treatment of NOD mice with ACK2 led to immediate hypersensitivity responses that required the coadministration of Benadryl to prevent anaphylaxis. Under these conditions, there was transient reversal of hyperglycemia in ∼60% of mice, however, a similar transient reduction in hyperglycemias was observed in control Ig plus Benadryl or Benadryl only-treated mice suggesting a transient effect on blood glucose because of the antihistamine (Fig. 3B). Thus, targeting c-Kit, either by PLX647 or anti-c-Kit mAb, was not sufficient to block disease progression.
Fig. 3.
Effect of PLX647 and anti-c-Kit treatments on diabetic mice. (A) New onset diabetic NOD mice were fed with mouse chow containing PLX647 (n = 9, 600 mg/kg of chow) or control chow (n = 6). Percentages of diabetic mice are shown. (B) New onset diabetic NOD mice received anti-c-Kit mAb (ACK2, 1 mg/mouse/day, i.v.) and Benadryl injection (30 mg/kg, i.p.) for 10 days (gray shaded area) (n = 11). Control mice received either rat IgG2b isotype plus Benadryl (n = 8) or Benadryl alone (n = 8).
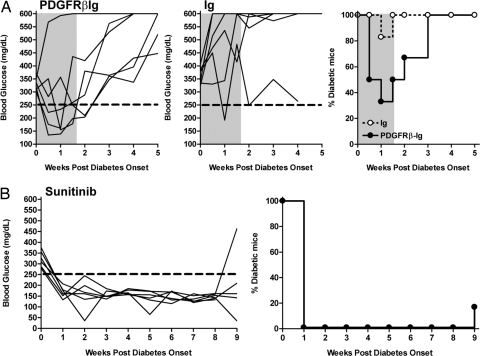
To test the effect of PDGF blockade, we generated a PDGFRβIg fusion protein. Specific inhibition of PDGFR signaling through PDGF neutralization was verified in vitro using NIH 3T3 cells (data not shown). As shown in Fig. 4A, diabetes remission was noted in five of six mice treated with PDGFRβIg for 10 days, whereas only one of six mice treated with control Ig transiently returned to normal glycemia. After cessation of therapy, all of the PDGFRβIg-treated mice relapsed to diabetes within 2 weeks. Unfortunately, continued treatment with the PDGFRβIg was not feasible as the human protein was quite immunogenic and the mice all developed a potent antibody response against the foreign protein.
Fig. 4.
Effect of PDGF neutralization and sunitinib treatment on diabetic mice. (A) New onset diabetic NOD mice received either PDGFRβIg fusion molecule (n = 6) or control human Ig (n = 6) at the dose of 200 μg/mouse/day, i.p., for 10 days (gray shaded area). Individual glucose readings and percentages of diabetic mice are shown. (B) Sunitinib treatment (gavage, 2 mg/mouse) of NOD mice (n = 6) was initiated at the time of disease onset and continued for 5 weeks.
Finally, we tested sunitinib, a small molecule potently inhibiting c-Kit, PDGFR and c-Fms (29) in an attempt to look directly at c-Kit and PDGFR kinases, which had shown some effect individually. As seen in Table S2, unlike PLX647, sunitinib strongly inhibited PDGFR-β. In this regard, treatment of diabetic mice with sunitinib resulted in remission of disease in 100% of treated mice within 1 week after initiation of therapy (Fig. 4B). Only one of six treated mice relapsed to diabetes within the continuous 9-week treatment. The suppressive effects continued for the duration of therapy, suggesting that efficacy did not depend on blocking Abl (or Lck) but is most likely targeting PDGFR, and to some extent c-Kit. Taken together, these data strongly suggest that short-term imatinib or sunitinib treatments affect the inflammatory response involved in the development and progression of diabetes in NOD mice.
Discussion
Type 1 diabetes remains an intractable disease with a limited number of treatment options that can reverse disease once diagnosed. In this study, we demonstrate that treatment with imatinib, an anticancer drug used to treat patients with CML and other tumors, prevented and reversed the development of autoimmune diabetes in the NOD mouse. This rapid and robust effect is a significant result given the paucity of existing treatments to induce remission in overtly diabetic mice (23). The immunosuppressive activity is reminiscent of recent case reports in patients with rheumatologic and other autoimmune diseases and a collagen-induced mouse model of rheumatoid arthritis. However, one unexpected observation was that limiting treatment to 8–10 weeks after disease onset was sufficient to reverse diabetes and induce long-term remission consistent with reestablishment of tolerance in these NOD mice. This observation provides a new perspective for the use of this kinase inhibitor or other similar drugs for the treatment of type 1 diabetes or other autoimmune diseases.
From a mechanistic point of view, the pleiotropic effect of the drug on multiple kinases had made it difficult to determine the biochemical basis of imatinib's efficacy. In fact, in the study by Paniagua et al., the investigators showed the marked effects of imatinib on several kinases (13); however, the precise mechanism of action of the drug in this and other models remained elusive. Because imatinib inhibits both c-Abl and Lck kinases, which are expressed in both B and T cells of the adaptive immune system, it seemed possible that these kinases might be involved as primary imatinib targets. Abl kinase is functional in both B and T cells, which have been shown to be critically involved in NOD diabetes (30). Moreover, c-Abl has been implicated in T cell activation and thymocyte development (31). Furthermore, imatinib has been shown to inhibit Lck (6), although to a lower extent than Abl. In vitro, imatinib inhibits T cell proliferation likely because of reduced phosphorylation of ZAP-70, LAT (24), Lck, and ERK1/2, and reduced levels of activated NFκB (12). However, in the current study, the imatinib effect did not appear to express through direct function on B or T cells. Imatinib treatment did not significantly alter insulitis, T cell number, or antigen-specific T cell proliferation. In addition, adoptive transfer of spleen cells from imatinib-treated mice into (untreated) severe combined immunodeficiency (SCID) mice resulted in a similar or only slightly delayed development of diabetes compared with spleen cells from oil-treated mice (data not shown). Finally, imatinib did not alter CD25 or Foxp3 expression in CD4+ Tregs in treated mice. The significant effects of imatinib on T cells in vitro likely reflect the relatively high concentrations of imatinib used (13). In fact, in vivo studies showed that imatinib treatment did not affect primary T cell responses (32, 33) or T cell function in patients treated with imatinib (34, 35). Thus, it would appear that T cells are not the primary target of imatinib activity.
The observation that imatinib weakly modulated IFN-γ expression in T cells (Fig. 2D) is consistent with a recent study (33) suggesting that drug treatment might decrease effector function through indirect effects on inflammation or non-T cells such as APCs. In this regard, some studies had suggested that imatinib negatively affected DC numbers and function (10, 36). However, 2 weeks of imatinib did not alter DC numbers or expression of MHC class II, B7–2 and IL-12p40 (37) (Fig. S2) or alter islet antigen processing and presentation by DCs (Fig. 2C).
Besides Abl and Lck, imatinib potently inhibits c-Kit [CD117, stem cell factor (SCF) receptor]. Mast cells express high levels of c-Kit and can have negative and positive immunomodulatory functions (38). Increasing evidence points to a role of mast cells in the development of autoimmune diseases such as multiple sclerosis, Guillain-Barré syndrome, rheumatoid arthritis, or T1D (39). Interestingly, Geoffrey et al. reported that treatment of diabetic BB rats with cromolyn delayed disease onset (40). Imatinib has been shown to inhibit SCF-induced activation of mast cell in vitro (13). Initial efforts to use an anti-c-Kit antibody, ACK2, in NOD mice resulted in immediate anaphylaxis and mouse death, an observation that was not observed in C57BL/6 mice (data not shown). Thus, to pursue treatment with this regimen, a single dose of the antihistamine, Benadryl, was administered before ACK2 administration. In fact, the combination therapy transiently reversed diabetes. However, treatment of new-onset diabetic mice with the antihistamine alone was sufficient to induce the transient remission in a significant number of mice. To further test the potential role of c-Kit in the long-term effects of imatinib, we examined the effect of PLX647, a c-Kit/c-Fms inhibitor, in the new-onset diabetes model (Table S2). This drug showed relatively weak efficacy in reversing diabetes compared with imatinib, suggesting that c-Kit is not the primary imatinib kinase target responsible for long-term reversal of T1D.
In contrast, we found that PDGF neutralization was very potent at reversing T1D compared with control Ig. Treatment of mice with a soluble form of PDGF receptor β rapidly led to normal glycemia that in a few animals lasted for weeks beyond the treatment. It should be noted that the majority of PDGFR mice relapsed. Unfortunately, continued treatment did not prolong the therapeutic activity of the biologic. Different reasons may account for the suboptimal effect of this therapy. First, as noted above, the soluble rat receptor was linked to a human Fc. This chimeric molecule was highly immunogenic and induced high titered, neutralizing antibodies (data not shown). Thus, continued treatment was unlikely to be efficacious. Second, the PDGFRβIg selectively blocks PDGF-BB but not the other dimers including PDGF-AA or PDGF-AB, molecules that would be inhibited by imatinib and sunitinib where the IC50s for PDGFR-A and B are 0.8 and 0.08 nM, respectively (41). Therefore, we turned to another PDGF inhibitor, sunitinib (Table S2). This drug was equally effective as imatinib in reversing diabetes. Although sunitinib also inhibits c-Kit, VEGFR, FLT3, and c-Fms, the combination of the results with the various biologic and small-molecule inhibitors suggest that inhibition of Abl, c-Fms, c-Kit, and Lck were not crucial to cure the disease.
PDGFs and PDGFRs (PDGFRα and β homo- and heterodimers) are widely expressed in diverse tissues, including the immune system, and have been implicated in pathological cell proliferation in the context of various diseases including fibrosis or tumor growth (42). In this regard, encouraging results have been achieved using α1-antitrypsin (AAT), a serine proteinase inhibitor that exhibits various antiinflammatory effects, in the context of islet allograft (43) or in the prevention of T1D in NOD mice (44). Increasing data suggest that the nonspecific inflammatory response is a key to the progression of diabetes promoting insulin resistance and IL-1-dependent β cell death. In this regard, we note that Hagerkvist et al. recently showed that imatinib ameliorates type 2 diabetes in rats by lowering insulin resistance (45). However, in a related study (22), the authors concluded that imatinib treatment preserved β cell viability through an Abl-dependent inhibition of NFκB. Our studies suggest that an Abl-independent mechanism of action is a key to the affects of imatinib in the T1D setting. Indeed, sunitinib, which inhibits c-Kit, PDGFR, and c-Fms, but not Abl, was as efficient as imatinib in inducing rapid remission from established diabetes (Fig. 4B). However, it remains possible that Abl inhibition might be responsible, at least in part, for the unique long-term efficacy after imatinib withdrawal or, in fact, plays an independent role in autoimmune diabetes in this model. It will be necessary to test abl-specific agents to address this directly. This possibility is consistent with increasing evidence of a role for B cells in long-term remission in NOD mice (30).
In summary, the development of small-molecule kinase inhibitors has revolutionized the treatment of cancer. Growing data suggest that these same drugs may have important applications in the treatment of autoimmune diseases. The findings in this study that both imatinib and sunitinib can reverse diabetes in new-onset T1D mice, combined with the fact that prolonged remission does not require continuous treatment and otherwise generally good safety profile (2, 46), suggest that selective kinase inhibitors may prove to be an important new therapeutic for clinical treatment of diabetes and other autoimmune diseases.
Materials and Methods
Mice.
Female NOD and NOD.SCID mice were purchased from Taconic. NOD.BDC2.5 TCR Tg mice (47) were provided by C. Benoist and D. Mathis (Harvard Medical School, Boston, Massachusetts). NOD.BDC2.5.Thy1.1 mice were derived in our laboratory. NOD.BDC2.5.Thy1.1.Yeti mice have been described previously (27). All mice were housed in a pathogen-free facility at the University of California, San Francisco. All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of California, San Francisco.
Drugs and Treatments
Imatinib.
Imatinib mesylate (Gleevec) tablets were purchased from Novartis, and ground and suspended in peanut oil to a concentration of 5 mg/ml. Mice were treated by gavage. Different timing and doses of imatinib administration were compared to develop an efficacious drug dosage that was in the range of human therapy. Specifically, different doses (100 mg/kg, 50 mg/kg or a constant dose of 1.5 mg/mouse or 3 mg/mouse) had similar effects on diabetes prevention and remission. A daily administration resulted in more reproducible results than every-other-day administration. Thus, for convenience, mice were routinely treated daily (Monday through Friday) with a single dose of 1.5 mg/mouse (0.3 ml). Control mice received 0.3 ml of oil only.
PLX647.
Rodent Chow Containing PLX647 (600 mg of PLX647 per kg of chow, Research Diets) was provided by Plexxikon. Treatment consisted in replacing regular chow by PLX647 chow (average daily dose of PLX647 is 60 mg/kg of mouse body weight). PLX647 is a selective inhibitor of c-Kit (IC50, 13 nM) and c-Fms (IC50, 28 nM) with selectivity vs. PDGFRβ (IC50, 460 nM).
Sunitinib.
Sunitinib malate (Sutent) capsules were purchased from Pfizer. Sunitinib was suspended in peanut oil to a concentration of 10 mg/ml. Mice were treated daily (Monday to Friday) by gavage with a constant dose of 2 mg/mouse (0.2 ml).
Anti-c-Kit mAb.
Anti-mouse c-Kit (CD117, ACK2 clone) and isotype control rat IgG2b anti-KLH (LTF-2 clone) mAbs were produced from hybridoma supernatants and purified using MEP HyperCel. Mice were treated daily (i.v.) with 1 mg of mAb. Benadryl (Baxter Healthcare) was given i.p. (30 mg/kg) 15 min before each mAb injection.
PDGFRβIg.
Rat PDGFRβ extracellular domain fused to human IgG1 Fc was cloned from the plasmid pDelta-CMV-PDGFRβIg-PolyA (a gift from Dr. Ralf Weiskirchen, Institute of Clinical Chemistry and Pathobiochemistry, RWTH-University Hospital, Aachen, Germany) into the plasmid pCEP4 (catalog (cat.) no. V044–50, Invitrogen) using EcoRI and XbaI (3105 bp). Soluble PDGFRβIg protein was expressed in HEK 293 Ebna cells and purified from supernatant using Protein A Sepharose 4 Fast Flow, (GE Healthcare Life Sciences) and finalized in PBS. OKT3γ1(Ala-Ala), a humanized IgG1 version of OKT3 (anti-human CD3) (48) was produced and purified in our laboratory and was used as control. Mice were treated daily (i.p.) with 0.2 μg of PDGFRβIg or OKT3γ1 (Ala-Ala).
Assessment of Diabetes and Insulitis.
Blood glucose levels were measured with a Lifescan glucose meter (One Touch II; Lifescan). Mice were considered diabetic after two consecutive measurements >250 mg/dl. Severity of insulitis was performed as previously described (30). Insulitis index (I) was calculated as previously described (49).
Adoptive Transfer.
For in vivo proliferation assays, BDC2.5 spleen and lymph node cells were depleted of CD25+ cells, labeled with 2.5 μM CFSE, and one million cells were transferred into NOD mice by retroorbital injection. For in vivo assessment of IFN-γ production, CD4+CD25−YFP− T cells from NOD.BDC2.5.Th1.1.Yeti mice were sorted on a MoFlo cytometer high speed cell sorter (DakoCytomation, Beckman Coulter) to ≥99% purity. One million cells were subsequently transferred into NOD mice by retroorbital injection.
Statistical Analysis.
All statistical analyses were performed using GraphPad Prism version 5.00. Statistical comparison of insulitis index, thymidine incorporation, CFSE dilution, and cell marker expression were performed using an unpaired two-tailed Mann-Whitney U test. Values of P ≤ 0.05 were considered significant.
Supplementary Material
Acknowledgments.
The authors thank P. Koudria for technical assistance. We thank H. Bour-Jordan and members of the Bluestone laboratory for helpful discussions. We thank Dr. Richard Locksley for generously providing the NOD.Yet40 mice. This work was supported by National Institutes of Health Grants R01 AI50834, R37 AI46643, and N01 AI15416 (to J.B.), P30 DK63720 (to J.B.), and the Juvenile Diabetes Research Foundation (JDRF) (3-2006-710 to C.L.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0810246105/DCSupplemental.
References
- 1.Druker BJ, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 2.Druker BJ, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 3.Buchdunger E, et al. Abl protein-tyrosine kinase inhibitor STI571 inhibits in vitro signal transduction mediated by c-kit and platelet-derived growth factor receptors. J Pharmacol Exp Ther. 2000;295:139–145. [PubMed] [Google Scholar]
- 4.Dewar AL, et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005;105:3127–3132. doi: 10.1182/blood-2004-10-3967. [DOI] [PubMed] [Google Scholar]
- 5.Guo J, et al. Inhibition of phosphorylation of the colony-stimulating factor-1 receptor (c-Fms) tyrosine kinase in transfected cells by ABT-869 and other tyrosine kinase inhibitors. Mol Cancer Ther. 2006;5:1007–1013. doi: 10.1158/1535-7163.MCT-05-0359. [DOI] [PubMed] [Google Scholar]
- 6.Fabian MA, et al. A small molecule-kinase interaction map for clinical kinase inhibitors. Nat Biotechnol. 2005;23:329–336. doi: 10.1038/nbt1068. [DOI] [PubMed] [Google Scholar]
- 7.Joensuu H, Dimitrijevic S. Tyrosine kinase inhibitor imatinib (STI571) as an anticancer agent for solid tumours. Ann Med. 2001;33:451–455. doi: 10.3109/07853890109002093. [DOI] [PubMed] [Google Scholar]
- 8.Pardanani A, et al. Imatinib for systemic mast-cell disease. Lancet. 2003;362:535–536. doi: 10.1016/s0140-6736(03)14115-3. [DOI] [PubMed] [Google Scholar]
- 9.Pardanani A, et al. Imatinib therapy for hypereosinophilic syndrome and other eosinophilic disorders. Blood. 2003;101:3391–3397. doi: 10.1182/blood-2002-10-3103. [DOI] [PubMed] [Google Scholar]
- 10.Boissel N, et al. Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia. 2004;18:1656–1661. doi: 10.1038/sj.leu.2403474. [DOI] [PubMed] [Google Scholar]
- 11.Wolf AM, et al. The kinase inhibitor imatinib mesylate inhibits TNF-{alpha} production in vitro and prevents TNF-dependent acute hepatic inflammation. Proc Natl Acad Sci USA. 2005;102:13622–13627. doi: 10.1073/pnas.0501758102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dietz AB, et al. Imatinib mesylate inhibits T-cell proliferation in vitro and delayed-type hypersensitivity in vivo. Blood. 2004;104:1094–1099. doi: 10.1182/blood-2003-12-4266. [DOI] [PubMed] [Google Scholar]
- 13.Paniagua RT, et al. Selective tyrosine kinase inhibition by imatinib mesylate for the treatment of autoimmune arthritis. J Clin Invest. 2006;116:2633–2642. doi: 10.1172/JCI28546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koyama K, et al. Imatinib mesylate both prevents and treats the arthritis induced by type II collagen antibody in mice. Mod Rheumatol. 2007;17:306–310. doi: 10.1007/s10165-007-0592-9. [DOI] [PubMed] [Google Scholar]
- 15.Sadanaga A, et al. Amelioration of autoimmune nephritis by imatinib in MRL/lpr mice. Arthritis Rheum. 2005;52:3987–3996. doi: 10.1002/art.21424. [DOI] [PubMed] [Google Scholar]
- 16.Miyachi K, et al. Efficacy of imatinib mesylate (STI571) treatment for a patient with rheumatoid arthritis developing chronic myelogenous leukemia. Clin Rheumatol. 2003;22:329–332. doi: 10.1007/s10067-003-0716-3. [DOI] [PubMed] [Google Scholar]
- 17.Eklund KK, Joensuu H. Treatment of rheumatoid arthritis with imatinib mesylate: Clinical improvement in three refractory cases. Ann Med. 2003;35:362–367. doi: 10.1080/07853890310001339. [DOI] [PubMed] [Google Scholar]
- 18.Miyagawa S, Fujimoto H, Ko S, Hirota S, Kitamura Y. Improvement of psoriasis during imatinib therapy in a patient with a metastatic gastrointestinal stromal tumour. Br J Dermatol. 2002;147:406–407. doi: 10.1046/j.1365-2133.2002.497217.x. [DOI] [PubMed] [Google Scholar]
- 19.Eklund KK, Remitz A, Kautiainen H, Reitamo S, Leirisalo-Repo M. Three months treatment of active spondyloarthritis with imatinib mesylate: An open-label pilot study with six patients. Rheumatology (Oxford) 2006;45:1573–1575. doi: 10.1093/rheumatology/kel365. [DOI] [PubMed] [Google Scholar]
- 20.Magro F, Costa C. Long-standing remission of Crohn's disease under imatinib therapy in a patient with Crohn's disease. Inflamm Bowel Dis. 2006;12:1087–1089. doi: 10.1097/01.mib.0000232468.15950.34. [DOI] [PubMed] [Google Scholar]
- 21.Anderson MS, Bluestone JA. The NOD mouse: A model of immune dysregulation. Annu Rev Immunol. 2005;23:447–485. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 22.Hagerkvist R, Sandler S, Mokhtari D, Welsh N. Amelioration of diabetes by imatinib mesylate (Gleevec): Role of beta-cell NF-kappaB activation and anti-apoptotic preconditioning. FASEB J. 2007;21:618–628. doi: 10.1096/fj.06-6910com. [DOI] [PubMed] [Google Scholar]
- 23.Shoda LK, et al. A comprehensive review of interventions in the NOD mouse and implications for translation. Immunity. 2005;23:115–126. doi: 10.1016/j.immuni.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Seggewiss R, et al. Imatinib inhibits T-cell receptor-mediated T-cell proliferation and activation in a dose-dependent manner. Blood. 2005;105:2473–2479. doi: 10.1182/blood-2004-07-2527. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman SM, et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc Natl Acad Sci USA. 2003;100:8384–8388. doi: 10.1073/pnas.0932778100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Judkowski V, et al. Identification of MHC class II-restricted peptide ligands, including a glutamic acid decarboxylase 65 sequence, that stimulate diabetogenic T cells from transgenic BDC2.5 nonobese diabetic mice. J Immunol. 2001;166:908–917. doi: 10.4049/jimmunol.166.2.908. [DOI] [PubMed] [Google Scholar]
- 27.Tang Q, et al. Visualizing regulatory T cell control of autoimmune responses in nonobese diabetic mice. Nat Immunol. 2006;7:83–92. doi: 10.1038/ni1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Czechowicz A, Kraft D, Weissman IL, Bhattacharya D. Efficient transplantation via antibody-based clearance of hematopoietic stem cell niches. Science. 2007;318:1296–1299. doi: 10.1126/science.1149726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murray LJ, et al. SU11248 inhibits tumor growth and CSF-1R-dependent osteolysis in an experimental breast cancer bone metastasis model. Clin Exp Metastasis. 2003;20:757–766. doi: 10.1023/b:clin.0000006873.65590.68. [DOI] [PubMed] [Google Scholar]
- 30.Bour-Jordan H, et al. Constitutive expression of B7–1 on B cells uncovers autoimmunity toward the B cell compartment in the nonobese diabetic mouse. J Immunol. 2007;179:1004–1012. doi: 10.4049/jimmunol.179.2.1004. [DOI] [PubMed] [Google Scholar]
- 31.Gu JJ, Zhang N, He YW, Koleske AJ, Pendergast AM. Defective T cell development and function in the absence of abelson kinases. J Immunol. 2007;179:7334–7343. doi: 10.4049/jimmunol.179.11.7334. [DOI] [PubMed] [Google Scholar]
- 32.Mumprecht S, Matter M, Pavelic V, Ochsenbein AF. Imatinib mesylate selectively impairs expansion of memory cytotoxic T cells without affecting the control of primary viral infections. Blood. 2006;108:3406–3413. doi: 10.1182/blood-2006-04-018705. [DOI] [PubMed] [Google Scholar]
- 33.Leder C, Ortler S, Seggewiss R, Einsele H, Wiendl H. Modulation of T-effector function by imatinib at the level of cytokine secretion. Exp Hematol. 2007;35:1266–1271. doi: 10.1016/j.exphem.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 34.Bocchia M, et al. Imatinib does not impair specific antitumor T-cell immunity in patients with chronic myeloid leukemia. Leukemia. 2005;20:142–143. doi: 10.1038/sj.leu.2404029. [DOI] [PubMed] [Google Scholar]
- 35.Chen CI, Maecker HT, Lee PP. Development and dynamics of robust T cell responses to CML under imatinib treatment. Blood. 2008;111:5342–5349. doi: 10.1182/blood-2007-12-128397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Appel S, et al. Effects of imatinib on monocyte-derived dendritic cells are mediated by inhibition of nuclear factor-kappaB and Akt signaling pathways. Clin Cancer Res. 2005;11:1928–1940. doi: 10.1158/1078-0432.CCR-04-1713. [DOI] [PubMed] [Google Scholar]
- 37.Reinhardt RL, Hong S, Kang SJ, Wang ZE, Locksley RM. Visualization of IL-12/23p40 in vivo reveals immunostimulatory dendritic cell migrants that promote Th1 differentiation. J Immunol. 2006;177:1618–1627. doi: 10.4049/jimmunol.177.3.1618. [DOI] [PubMed] [Google Scholar]
- 38.Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: Negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sayed BA, Christy A, Quirion MR, Brown MA. The master switch: The role of mast cells in autoimmunity and tolerance. Annu Rev Immunol. 2008;26:705–739. doi: 10.1146/annurev.immunol.26.021607.090320. [DOI] [PubMed] [Google Scholar]
- 40.Geoffrey R, et al. Evidence of a functional role for mast cells in the development of type 1 diabetes mellitus in the BioBreeding rat. J Immunol. 2006;177:7275–7286. doi: 10.4049/jimmunol.177.10.7275. [DOI] [PubMed] [Google Scholar]
- 41.Karaman MW, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 42.Betsholtz C. Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev. 2004;15:215–228. doi: 10.1016/j.cytogfr.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA. 2005;102:12153–12158. doi: 10.1073/pnas.0505579102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lu Y, et al. Alpha1-antitrypsin gene therapy modulates cellular immunity and efficiently prevents type 1 diabetes in nonobese diabetic mice. Hum Gene Ther. 2006;17:625–634. doi: 10.1089/hum.2006.17.625. [DOI] [PubMed] [Google Scholar]
- 45.Hagerkvist R, Makeeva N, Elliman S, Welsh N. Imatinib mesylate (Gleevec) protects against streptozotocin-induced diabetes and islet cell death in vitro. Cell Biol Int. 2006;30:1013–1017. doi: 10.1016/j.cellbi.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 46.Ribeiro AL, et al. An evaluation of the cardiotoxicity of imatinib mesylate. Leuk Res. 2008;32:1809–1814. doi: 10.1016/j.leukres.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Katz JD, Wang B, Haskins K, Benoist C, Mathis D. Following a diabetogenic T cell from genesis through pathogenesis. Cell. 1993;74:1089–1100. doi: 10.1016/0092-8674(93)90730-e. [DOI] [PubMed] [Google Scholar]
- 48.Herold KC, et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes. 2005;54:1763–1769. doi: 10.2337/diabetes.54.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen W, et al. Insulin-like growth factor (IGF)-I/IGF-binding protein-3 complex: Therapeutic efficacy and mechanism of protection against type 1 diabetes. Endocrinology. 2004;145:627–638. doi: 10.1210/en.2003-1274. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.