Abstract
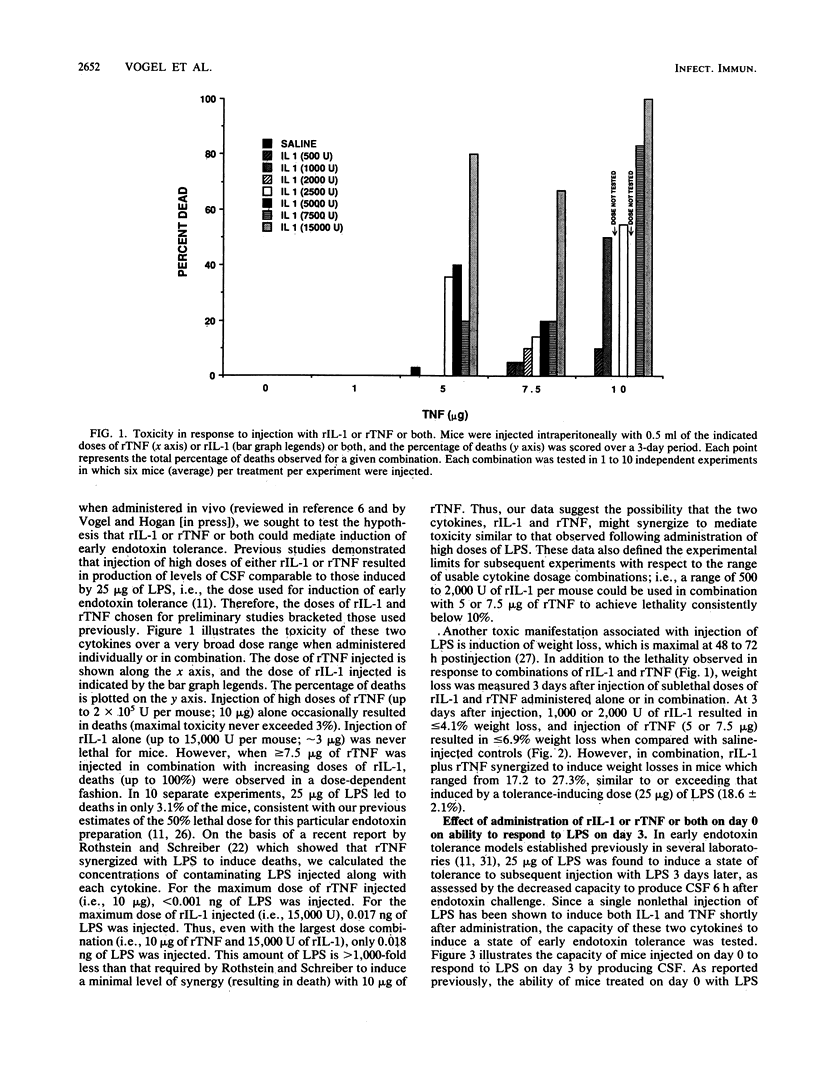
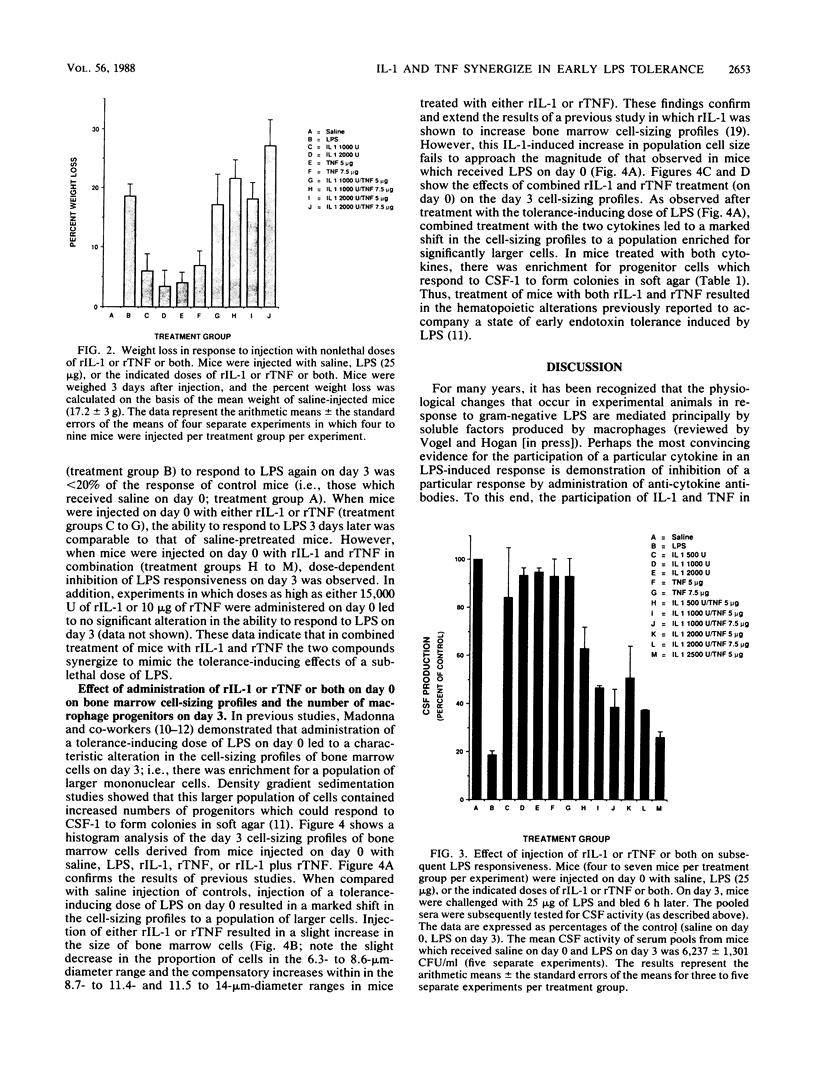
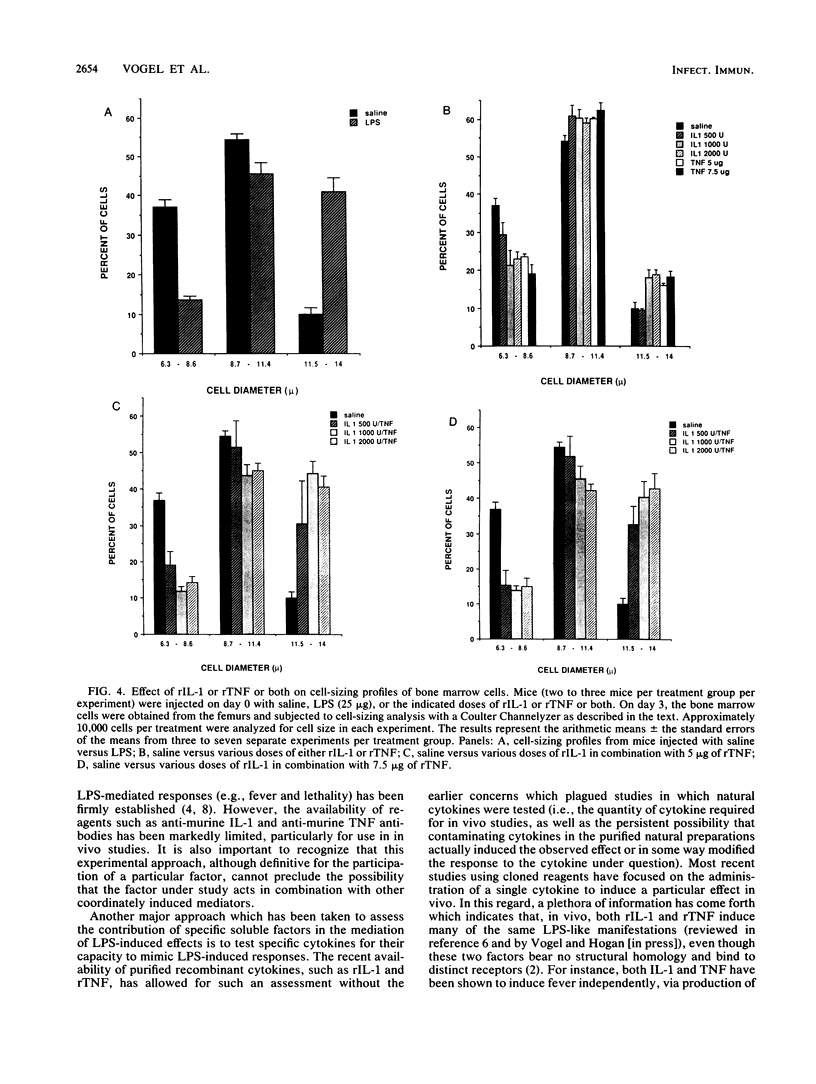
Endotoxin, the lipopolysaccharide (LPS) derived from gram-negative bacteria, invokes a wide range of responses in susceptible hosts. It is known that virtually all responses to LPS are mediated by the action of macrophage-derived cytokines (such as interleukin-1 [IL-1], tumor necrosis factor [TNF], and others) which are produced principally by macrophages and maximally within several hours of LPS administration. One manifestation of LPS administration which is not well understood is the phenomenon of "early endotoxin tolerance." In response to a single sublethal injection of LPS, experimental animals become refractory to challenge with a homologous or heterologous LPS preparation 3 to 4 days later. Animals rendered tolerant exhibit mitigated toxicity and a reduced capacity to produce circulating cytokines (i.e., colony-stimulating factor or interferon) in response to the challenge LPS injection. Previous studies have also shown that this state of transient, acquired hyporesponsiveness to LPS is accompanied by a marked increase in the size of cells in the bone marrow which are enriched in numbers of macrophage progenitors. In this study, we examined the capacity of recombinant IL-1 or recombinant TNF or both to induce early endotoxin tolerance and its associated hematopoietic changes. Neither cytokine alone was able to mimic LPS for induction of tolerance. Combined administration of recombinant IL-1 and recombinant TNF doses which were not toxic when administered individually led to synergistic toxicity (as assessed by death or weight loss). However, within a nontoxic range, the two cytokines synergized to induce a significant reduction in the capacity to produce colony-stimulating factor in response to LPS, as well as the characteristic increase in bone marrow cell size and macrophage progenitors shown previously to be associated with LPS-induced tolerance.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck G., Habicht G. S., Benach J. L., Miller F. Interleukin 1: a common endogenous mediator of inflammation and the local Shwartzman reaction. J Immunol. 1986 Apr 15;136(8):3025–3031. [PubMed] [Google Scholar]
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Biology of interleukin 1. FASEB J. 1988 Feb;2(2):108–115. [PubMed] [Google Scholar]
- Dinarello C. A., Bodel P. T., Atkins E. The role of the liver in the production of fever and in pyrogenic tolerance. Trans Assoc Am Physicians. 1968;81:334–344. [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Wolff S. M., Bernheim H. A., Beutler B., Cerami A., Figari I. S., Palladino M. A., Jr, O'Connor J. V. Tumor necrosis factor (cachectin) is an endogenous pyrogen and induces production of interleukin 1. J Exp Med. 1986 Jun 1;163(6):1433–1450. doi: 10.1084/jem.163.6.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna G. S., Peterson J. E., Ribi E. E., Vogel S. N. Early-phase endotoxin tolerance: induction by a detoxified lipid A derivative, monophosphoryl lipid A. Infect Immun. 1986 Apr;52(1):6–11. doi: 10.1128/iai.52.1.6-11.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madonna G. S., Vogel S. N. Early endotoxin tolerance is associated with alterations in bone marrow-derived macrophage precursor pools. J Immunol. 1985 Dec;135(6):3763–3771. [PubMed] [Google Scholar]
- Madonna G. S., Vogel S. N. Induction of early-phase endotoxin tolerance in athymic (nude) mice, B-cell-deficient (xid) mice, and splenectomized mice. Infect Immun. 1986 Sep;53(3):707–710. doi: 10.1128/iai.53.3.707-710.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Mortensen R. F., Shapiro J., Lin B. F., Douches S., Neta R. Interaction of recombinant IL-1 and recombinant tumor necrosis factor in the induction of mouse acute phase proteins. J Immunol. 1988 Apr 1;140(7):2260–2266. [PubMed] [Google Scholar]
- Movat H. Z., Cybulsky M. I., Colditz I. G., Chan M. K., Dinarello C. A. Acute inflammation in gram-negative infection: endotoxin, interleukin 1, tumor necrosis factor, and neutrophils. Fed Proc. 1987 Jan;46(1):97–104. [PubMed] [Google Scholar]
- Neta R., Oppenheim J. J., Douches S. D. Interdependence of the radioprotective effects of human recombinant interleukin 1 alpha, tumor necrosis factor alpha, granulocyte colony-stimulating factor, and murine recombinant granulocyte-macrophage colony-stimulating factor. J Immunol. 1988 Jan 1;140(1):108–111. [PubMed] [Google Scholar]
- Neta R., Sztein M. B., Oppenheim J. J., Gillis S., Douches S. D. The in vivo effects of interleukin 1. I. Bone marrow cells are induced to cycle after administration of interleukin 1. J Immunol. 1987 Sep 15;139(6):1861–1866. [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Douches S. D., Kaufman E. N., Neta R. Induction of colony stimulating factor in vivo by recombinant interleukin 1 alpha and recombinant tumor necrosis factor alpha 1. J Immunol. 1987 Apr 1;138(7):2143–2148. [PubMed] [Google Scholar]
- Vogel S. N., English K. E., O'Brien A. D. Silica enhancement of murine endotoxin sensitivity. Infect Immun. 1982 Nov;38(2):681–685. doi: 10.1128/iai.38.2.681-685.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Moore R. N., Sipe J. D., Rosenstreich D. L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. I. In vivo studies. J Immunol. 1980 Apr;124(4):2004–2009. [PubMed] [Google Scholar]
- Wang A. M., Creasey A. A., Ladner M. B., Lin L. S., Strickler J., Van Arsdell J. N., Yamamoto R., Mark D. F. Molecular cloning of the complementary DNA for human tumor necrosis factor. Science. 1985 Apr 12;228(4696):149–154. doi: 10.1126/science.3856324. [DOI] [PubMed] [Google Scholar]
- Wankowicz Z., Megyeri P., Issekutz A. Synergy between tumour necrosis factor alpha and interleukin-1 in the induction of polymorphonuclear leukocyte migration during inflammation. J Leukoc Biol. 1988 Apr;43(4):349–356. doi: 10.1002/jlb.43.4.349. [DOI] [PubMed] [Google Scholar]
- Williams Z., Hertogs C. F., Pluznik D. H. Use of mice tolerant to lipopolysaccharide to demonstrate requirement of cooperation between macrophages and lymphocytes to generate lipopolysaccharide-induced colony-stimulating factor in vivo. Infect Immun. 1983 Jul;41(1):1–5. doi: 10.1128/iai.41.1.1-5.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngner J. S., Stinebring W. R. Interferon appearance stimulated by endotoxin, bacteria, or viruses in mice pre-treated with Escherichia coli endotoxin or infected with Mycobacterium tuberculosis. Nature. 1965 Oct 30;208(5009):456–458. doi: 10.1038/208456a0. [DOI] [PubMed] [Google Scholar]


