Abstract
Patients with cancer have circulating heterophile antibodies that agglutinate animal red cells via recognition of the mammalian cell surface sialic acid N-glycolylneuraminic acid (Neu5Gc), which was long considered an oncofetal antigen in humans. However, humans are genetically deficient in Neu5Gc production and instead metabolically accumulate Neu5Gc from dietary sources, particularly red meats and milk products. Moreover, mice with a human-like defect showed no alternate pathway for Neu5Gc synthesis and even normal humans express anti-Neu5Gc antibodies. We show here that human tumors accumulate Neu5Gc that is covalently attached to multiple classes of glycans. The paradox of human tumor Neu5Gc accumulation in the face of circulating anti-Neu5Gc antibodies was hypothesized to be due to facilitation of tumor progression by the resulting low-grade chronic inflammation. Indeed, murine tumors expressing human-like levels of Neu5Gc show accelerated growth in syngeneic mice with a human-like Neu5Gc deficiency, coincident with the induction of anti-Neu5Gc antibodies and increased infiltration of inflammatory cells. Transfer of polyclonal monospecific syngeneic mouse anti-Neu5Gc serum also enhanced growth of transplanted syngeneic tumors bearing human-like levels of Neu5Gc, with tumors showing evidence for antibody deposition, enhanced angiogenesis and chronic inflammation. These effects were suppressed by a cyclooxygenase-2 inhibitor, a drug type known to reduce human carcinoma risk. Finally, affinity-purified human anti-Neu5Gc antibodies also accelerate growth of Neu5Gc-containing tumors in Neu5Gc-deficient mice. Taken together, the data suggest that the human propensity to develop diet-related carcinomas is contributed to by local chronic inflammation, resulting from interaction of metabolically-accumulated dietary Neu5Gc with circulating anti-Neu5Gc antibodies.
Keywords: antibodies, COX-2, Neu5Gc, red meat, sialic acid
A prominent feature of malignant human tumors is the aberrant expression of cell-surface glycans, especially an enrichment of certain sialic acid containing antigens (for review see ref. 1). The majority of sialylated tumor markers in humans involve changes in presentation of the common human sialic acid N-acetylneuraminic acid (Neu5Ac). Another sialic acid called N-glycolylneuraminic acid (Neu5Gc) is abundant in most mammals, but not in normal humans, due to a deletion in the human gene coding for CMP-Neu5Ac hydroxylase (2, 3), the enzyme responsible for Neu5Gc biosynthesis. Despite no known alternative pathway for Neu5Gc biosynthesis in humans, there is evidence (primarily based on antibodies) that this sialic acid accumulates in human tumors, including colon carcinomas, retinoblastomas, breast cancers, and melanomas (4–8). Our data suggest that it might be explained by incorporation from dietary sources, predominantly red meats and milk products (8), foods that have also been epidemiologically linked to the increased cancer risk (9–17). We have shown that uptake of Neu5Gc into human tumor cells in vitro occurs primarily by macropinocytosis and delivery into the cytosolic compartment via a lysosomal transporter (18), and that this process is enhanced by high cell growth rates (19). Furthermore, hypoxic conditions in tumors can up-regulate expression of the lysosomal Sia transporter (20). Once it reaches the cytosolic compartment of human cells, the Neu5Gc molecule can be activated and incorporated into cell surface molecules as if it was originally made in the same cell (18). We therefore suggested that similar mechanisms might explain the apparent accumulation of Neu5Gc in tumor cells in vivo.
The epitope recognized by heterophile serum sickness “Hanganutziu-Deicher (HD)” antibodies involves Neu5Gc (21, 22). These antibodies were originally described in patients injected with animal serum. However, they were later detected in some disease-related human sera, without the patients having ever received animal sera. Prominent among these were cancer patients (23). More recently, we have shown that even normal humans can have high levels of anti-Neu5Gc antibodies (19, 24). Thus we have an apparent paradox wherein human tumors are claimed to accumulate Neu5Gc in the face of circulating anti-Neu5Gc antibodies.
One possible explanation is that the resulting low-grade chronic inflammation is insufficient to kill the tumor and instead facilitates tumor progression. Indeed, a potential tumor-promoting role for the adaptive immune system was described over 50 years ago and emphasized by Prehn (25–27), suggesting that a moderate anti-tumor immune reaction may not eliminate, but instead stimulate, tumor growth and survival. This concept of a bimodal response of the immune system to tumors is now regaining acceptance, and the stimulating effect of weak immune responses is thought to be mediated by growth-promoting and angiogenic signals originating from infiltrating innate immune cells (28–32). Indeed, many cancers are associated with chronic inflammation (29, 32–34), and there is good evidence from others that chronic activation of innate immune cells can promote carcinogenesis (30, 32). Similar roles have been suggested for adaptive immune cells (33–35), including evidence of antibodies generating inflammatory conditions and facilitating malignant progression in a mouse model of skin cancer (36). Based on these considerations, we have studied the non-human sialic acid Neu5Gc as a potential molecular link between diet, autoreactive antibodies, and the progression of human cancer.
Results
Human Malignancies Express Neu5Gc-Containing Glycans.
Use of an affinity-purified polyclonal monospecific anti-Neu5Gc chicken IgY antibody confirmed prior reports of the expression of the non-human sialic acid, Neu5Gc, in human tumor samples (8). Further studies (not shown) demonstrated anti-Neu5Gc IgY binding to 5/8 breast carcinomas, 3/3 ovarian carcinomas, 2/4 prostate carcinomas, 1/4 lung carcinomas, and 1/1 neuroblastomas from humans. Prior chemical evidence for human tumor Neu5Gc has been limited, primarily to detection of free Neu5Gc in acid hydrolysates of lipid extracts and mucins (37, 38). We chemically analyzed the Neu5Gc content of three such tumors (lung, pancreatic, ovarian), fractionating extracts into multiple glycan classes and studying the sialic acid profile by DMB-derivatization and HPLC analysis. We found that Neu5Gc was present at levels in the range of 1–4% of total sialic acids within each glycan class (Table 1), levels much higher than what we previously found in normal human tissues (8). Further analysis of the N-glycans by mass spectrometry provided direct evidence for covalently-bound Neu5Gc in these molecules from all three types of cancers (an example of N-glycan profiling is shown in supporting information (SI) Fig. S1).
Table 1.
Presence of Neu5Gc in pancreatic, breast, and ovary cancer tissue extracts
| Neu5Gc (% of total sialic acids) |
|||
|---|---|---|---|
| Glycosphingolipids | N-glycans | O-glycans | |
| Ovary (normal) | <0.25% | <0.25% | <0.25% |
| Ovarian cancer | 2.34% | 1.25% | 1.05% |
| Pancreatic cancer | 1.33% | 3.33% | <0.25% |
| Breast cancer | 2.43% | 1.03% | <0.25% |
Ovarian, pancreatic, and breast carcinoma extracts were fractionated into glycan classes, and Neu5Gc content was analyzed by DMB-derivatization and HPLC analysis, as described in SI Text.
Neu5Gc-Expressing Tumors in Neu5Gc-Deficient Mice Induce Antibodies and Grow Faster.
To model and test the hypothesis that chronic inflammation resulting from the combination of Neu5Gc in tumors and circulating anti-Neu5Gc antibodies can enhance human carcinoma progression, we turned to our recently generated Cmah−/− mice, with a human-like deficiency of Neu5Gc (39). Although such mice could accumulate Neu5Gc into a transgenic oncogene-induced mammary tumor (39), it took a long time and levels were much lower than seen in human tumors. This is presumably because of the much longer periods of time (years) during which human tumors can grow and accumulate Neu5Gc. The aggressive nature of such murine tumor models did not allow us to mimic the human-Neu5Gc loading over time, since the experiments had to be terminated within a month of tumor onset, due to the size of the developed tumors.
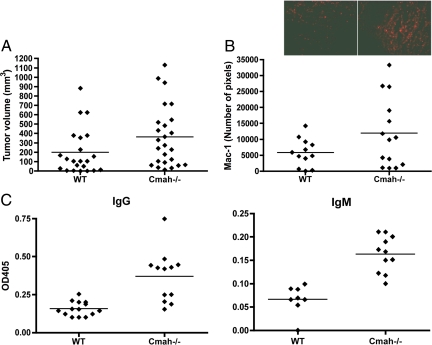
To better mimic the human condition, we therefore did further studies using selected C57BL/6 syngeneic mouse tumor lines, which express Neu5Gc at levels similar to those we found in human tumors. Syngeneic B16 melanoma cells (expressing approximately 5% Neu5Gc) were first inoculated s.c. into Cmah−/− or wild-type mice, to study the possible formation and role of anti-Neu5Gc antibodies in the Cmah−/− mice, in which a Neu5Gc-expressing tumor is expected to be seen as foreign. Wild-type mice on the other hand would not react as much to this syngeneic tumor cell line, as they already express Neu5Gc and thus cannot mount a major antibody immune response against it. There was a clear trend toward larger tumors in the Cmah−/− background (Fig. 1A, P = 0.06, as with all tumor growth experiments, there was scatter in the data). Furthermore, there was an increase in infiltration of inflammatory cells as shown by staining of Mac-1 positive cells (Fig. 1B). Sera from these tumor-bearing mice also showed spontaneous appearance of IgM and IgG Neu5Gc-specific antibodies, with levels varying amongst individual mice, but the overall result being highly significant (Fig. 1C). Such immune responses were primarily found in Cmah−/− mice compared to wild-type mice (Fig. 1C), supporting the hypothesis that tumor growth is enhanced by anti-Neu5Gc immune responses that can only occur in the null mice. Further detailed studies in the B16 melanoma system were not possible because of very rapid growth, causing ulceration and loss of tumor mass, and the individual variability of spontaneous anti-Neu5Gc responses. An additional difficulty is that intentional preimmunization of Cmah−/− mice with Neu5Gc-containing antigens gave variable immune responses in individual mice (data not shown).
Fig. 1.
Enhanced growth of Neu5Gc-expressing tumors in Cmah −/− mice with a human-like deficiency in Neu5Gc production. (A) Neu5Gc expressing B16 melanoma cells were injected s.c. into the flank of C57Bl6 wild-type or Cmah−/− mice. Tumors were removed from the flank, weighed, measured, and processed for immunostaining as described under Methods. Pooled data from four experiments, where each dot represents a single animal (P = 0.06). (B) Neu5Gc-expressing tumors growing in Cmah−/− mice show evidence of increased leukocyte infiltration by immunofluorescence staining using anti-Mac-1 antibody (see Inset for an example). There was increased Mac-1 staining of tumors grown in Cmah−/− mice compared to wild-type mice (P = 0.08). (C) Tumor-bearing mice show variable anti-Neu5Gc antibody response in serum collected at the time of tumor harvest. The binding specificity was examined using ELISA with human versus chimpanzee serum (Neu5Gc negative and positive respectively), as described in the Methods. Both anti-Neu5Gc IgG and IgM were significantly elevated in Cmah−/− mice (P < 0.05 and P < 0.005 respectively).
Generation of Polyclonal Anti-Neu5Gc-Specific Antibodies in Syngeneic Mice for Passive Transfer.
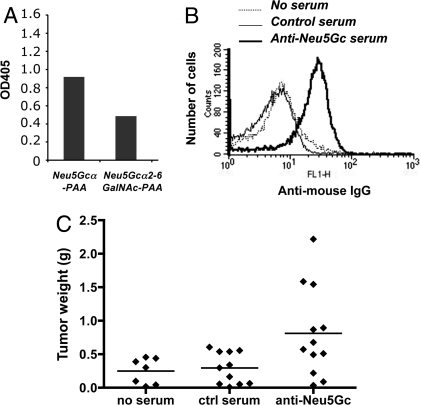
To better control further studies and to simultaneously confirm antibody involvement, we turned to the use of passive transfer of serum pooled from multiple immunized syngeneic mice. We generated a pool of syngeneic serum IgG specifically reactive with at least two different Neu5Gc-containing epitopes along with a well-matched control serum pool immunized and adsorbed in the identical manner (Fig. 2A). To elicit a broad polyclonal response covering many Neu5Gc-containing epitopes, we chose red blood cell (RBC) membranes ghosts from chimpanzees as the immunogen with human RBC ghosts as controls (these two cell types are extremely similar, with the major difference being the presence or absence of Neu5Gc) (40). To remove any resulting non-sialic acid dependent antibodies while retaining the Neu5Gc-reactive antibodies, RBC-immunized mouse sera were adsorbed repeatedly against human RBCs, until there was no more reactivity against the human cells. The resulting adsorbed chimpanzee RBC-immunized serum was indeed selectively reactive with Neu5Gc-containing epitopes (Fig. 2A), while the corresponding control serum was not (data not shown).
Fig. 2.
Generation and characterization of a polyclonal mouse anti-Neu5Gc serum for passive transfer studies. Anti-Neu5Gc antibodies were generated by immunization of Cmah−/− mice using cell membrane preparations from chimpanzee RBC or human RBC (control) in Freund's complete adjuvant. The resulting sera were then adsorbed against human RBCs. (A) Adsorbed mouse anti-Neu5Gc antisera show a polyclonal response against two different Neu5Gc-containing epitopes, as shown here by ELISA using microtiter plates coated with Neu5Gcα-polyacrylamide (Neu5GcαPAA) or Neu5Gcα2–6GalNAc-PAA, (see Methods for further details). (B) Adsorbed anti-Neu5Gc antibodies selectively react with MC-38 tumor cells, but there was no binding of adsorbed serum control to these cells. (C) Passively transferred anti-Neu5Gc antibodies stimulate the growth of transplanted Neu5Gc-positive syngeneic tumors. Cmah−/− mice were injected s.c. with MC-38 adenocarcinoma cells and on day 5 injected I.P. with syngeneic anti-Neu5Gc containing mouse serum. Data were pooled from two experiments, and each dot represents a single animal (P < 0.05).
Cyclooxygenase-2-Dependent Inflammation and Angiogenesis.
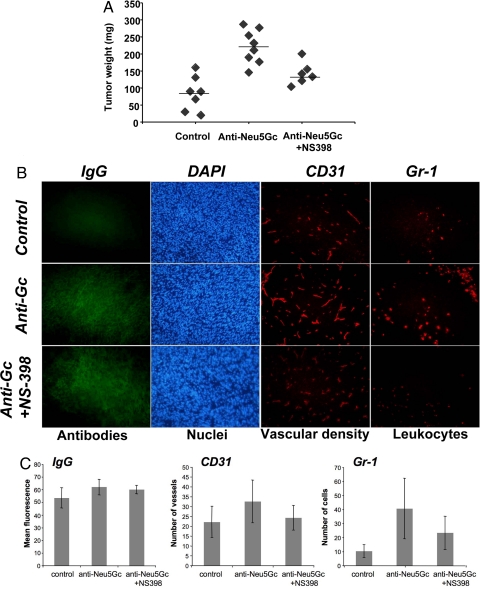
Exactly as predicted from earlier hypotheses on the bimodal responses of tumors to the immune system (25, 33, 34), we found that while a high dose of the anti-Neu5Gc antibody (passive transfer of 200 μl of immune but not control serum) was inhibitory for B16 tumor growth, a lower dose (20 μl of immune serum) stimulated growth (data not shown). The lower dose delivers 14.4 μg of mouse anti-Neu5Gc IgG into a mouse with an extracellular fluid volume of approximately 2 ml, i.e., a final concentration of antibodies within the range we found in some normal humans (24). As mentioned earlier, the rapid growth of B16 tumors made it difficult to follow the consequences in a reliable fashion. We therefore switched to another syngeneic tumor (the mouse colon adenocarcinoma MC-38), which also happens to express Neu5Gc in a range similar to that which we found in human tumors (1–4%). The anti-Neu5Gc serum pool recognized these syngeneic MC-38 tumor cells in flow cytometry, with no binding of the control serum (Fig. 2B). These tumor cells were injected into Cmah−/− mice, which were subsequently injected with 20 μl of control or anti-Neu5Gc containing serum on day 5–7. As hypothesized, we saw clearly enhanced growth of MC-38 tumor cells in the mice receiving the anti-Neu5Gc serum (Fig. 2C, P < 0.05 and Fig. 3A, P < 0.001).
Fig. 3.
Passive transfer of anti-Neu5Gc antibodies induces COX-2 dependent tumor growth, inflammation and increased vascular density. (A) Inhibition of antibody effects on tumor growth by the COX-2 inhibitor NS-398. Tumor-bearing Cmah−/− mice were injected i.p. with 10 mg NS-398 per kg of body weight every 3 days after injection of tumor cells. On day 14–18 tumors were harvested, weighed, and processed for immunohistochemistry, as described under Methods. Data from one representative experiment is shown, with each dot representing a single animal. There was a significant difference between control and anti-Neu5Gc serum treated mice (P < 0.001) and between NS-398-treated or untreated mice (both receiving anti-Neu5Gc serum), (P < 0.005). (B) Evidence of antibody deposition, increased vascular density, and leukocyte infiltration after passive transfer of adsorbed anti-Neu5Gc containing serum. Harvested tumors were analyzed by immunofluorescence using anti-mouse IgG (detecting Ig deposition), anti-CD31 (detecting blood vessels), or anti-Gr-1 (detecting leukocyte infiltration). (C) Positive staining of antibody deposition, angiogenesis, and leukocyte infiltration was quantified using Adobe Photoshop, analyzing three random high power fields of each tumor. Tumors from mice receiving anti-Neu5Gc antibodies displayed significantly increased IgG deposition (P < 0.05), Gr-1 staining (P < 0.01), and increased CD31 staining (P = 0.063). COX-2 inhibition using NS-398 reduced the vascular density (P = 0.058) and leukocyte infiltration (P = 0.052), but left IgG antibody deposition unaltered.
Increased tumor deposition of IgG was seen only in the Cmah−/− mice that received anti-Neu5Gc antibodies, but not in those receiving control serum (Fig. 3 B and C). Furthermore, tumors in the former mice showed increased infiltration of inflammatory cells and increased vascular density, indicating enhanced angiogenesis (Fig. 3 B and C). Many human tumors are known to have increased expression of cyclooxygenase-2 (COX-2), which can regulate the actions of the angiogenic factor vascular endothelial growth factor (VEGF), as well as multiple inflammatory pathways (41). Inhibition by a specific COX-2 inhibitor (NS-398) had no significant effect on IgG deposition in tumors, but reduced inflammation and angiogenesis in the anti-Neu5Gc antibody exposed tumors (Fig. 3B). Importantly, the effect on tumor growth was also abrogated, and the tumor weight in mice receiving NS-398 was reduced to control levels (Fig. 3A). This data also fit well with the known effects of aspirin and non-steroidal anti-inflammatory drugs (NSAIDs) such as selective cyclooxygenase-2 (COX-2) inhibitors, which have been shown to reduce cancer risk in humans (41–43).
Affinity-Purified Anti-Neu5Gc Antibodies from Humans Can Enhance Tumor Growth.
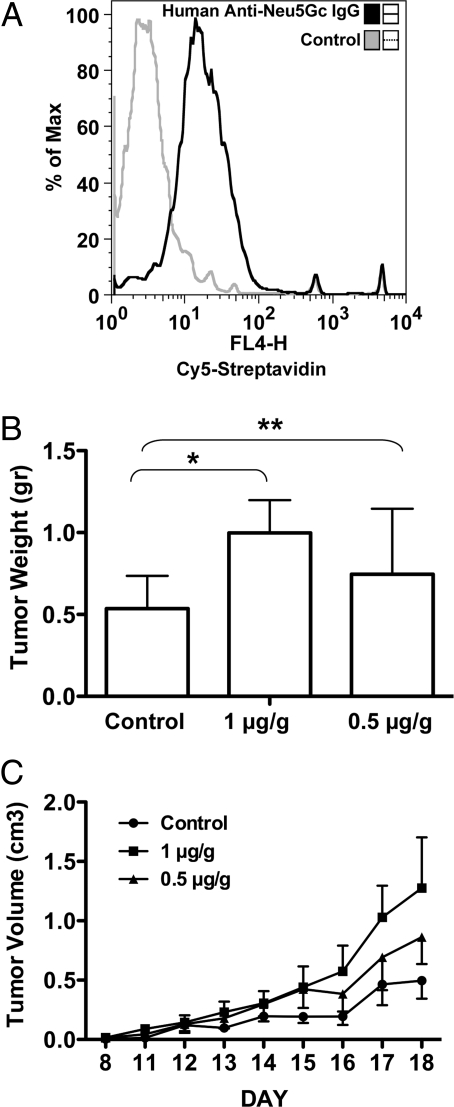
In a recent study, we showed that we could affinity-purify polyclonal monospecific anti-Neu5Gc antibodies from human serum (24). As shown in Fig. 4A, these specific antibodies could also react with the MC-38 mouse tumor cells that intrinsically express low levels of Neu5Gc. Similar to the mouse antibody data, we found that injection of high levels of affinity-purified human antibodies tended to inhibit the growth of the MC-38 tumor cells in the Cmah−/− null mice (data not shown). However, when used at intermediate dosage ranges (1 μg/g body weight), human anti-Neu5Gc antibodies caused a clear increase in the average weight of the tumors (Fig. 4B, P = 0.06 for the higher of the two doses used), while a lower dose (0.5 μg/g) did not. In this experiment, we also followed the tumor growth on a daily basis. As shown in Fig. 4C, the tumors exposed to the antibodies showed early growth rate acceleration, which accounted at least in part for the final average increase in tumor size. When this serial data were analyzed by a two-way ANOVA statistic, it showed a highly significant result for the difference in tumor volume from those in PBS-injected mice (P < 0.01 on day 18).
Fig. 4.
Human anti-Neu5Gc antibodies enhance tumor growth. (A) Affinity purified human anti-Neu5Gc IgG antibodies were biotinylated and binding to MC-38 cells (intrinsically expressing Neu5Gc) was detected with Cy5-streptavidin by flow cytometry. We used Cy5-streptavidin (without anti-Neu5Gc antibodies) as the control for binding. Data shown is representative of two independent experiments. (B) MC38 cells were injected s.c. into Cmah−/− mice. Five days later, affinity purified human anti-Neu5Gc antibodies in PBS were injected i.p. at 1 μg/g or 0.5 μg/g mouse body weight (n = 8 per group), control mice were injected with PBS (n = 7). On day 18, tumors were harvested and weighed, showing that mice receiving the higher antibody dose had larger tumors relative to the lower dose or control. (*, P = 0.06, **, P = 0.3 in an unpaired t test). (C) Tumor volumes were calculated from daily tumor measurements. The higher dose of antibodies promoted the growth of tumors (two-way-ANOVA; P < 0.01 on day 18) at a higher rate then the lower dose (P > 0.05 on day 18), compared to the control.
Discussion
Here we have provided chemical confirmation for the covalent addition of Neu5Gc to multiple classes of human tumor glycans and addressed a long-standing mystery in human tumor biology, that such increased Neu5Gc expression occurs in human tumors that are progressing in the face of an anti-Neu5Gc antibody response in the circulation. Knowing now that antibodies against Neu5Gc are much more common in normal humans than previously recognized (19, 24), we hypothesized that they contribute to tumor progression by stimulating inflammation via binding to Neu5Gc-positive tumor cells. The antibody deposition could then trigger infiltration and activation of inflammatory cells by activation of the complement system and/or the engagement of Fc receptors. Indeed, we demonstrate here that the combination of tumor-associated Neu5Gc and circulating anti-Neu5Gc antibodies promotes tumor growth, by inducing weak inflammation, causing infiltration of inflammatory cells and enhanced angiogenesis. These findings were further supported by COX-2 inhibition, which reduced the inflammation and decreased angiogenesis in the tumors. Thus, the paradoxical presence of increased Neu5Gc in human tumors in the face of an anti-Neu5Gc antibody response is likely because Neu5Gc incorporation confers a selective advantage to tumor cells that are best at accumulating Neu5Gc, due to the effects of the weak immune response in supporting early growth and angiogenesis. Importantly, these data are also congruent with the well-known epidemiological association of human cancers with consumption of Neu5Gc-rich foods such as red meat and milk (9–17), the decreased cancer risk associated with veganism (44), and the decreased cancer risk associated with the use of COX-2 inhibitors (41–43). The data are also consistent with the apparent rarity of such carcinomas in our closest evolutionary cousins the chimpanzees (45), who normally express Neu5Gc and thus do not make antibodies against it (8).
The modern focus on immunotherapy of cancer has tended to direct attention toward the potential role of the immune system in preventing and/or treating cancers (46, 47). Indeed, there is evidence that immune reactions against tumors are a mechanism of “host defense” (35), and this forms the basis for many popular approaches toward cancer immunotherapy (47). Thus, there is a need to explain the paradox of Neu5Gc accumulating on tumor cell surfaces in the face of circulating anti-Neu5Gc antibodies. In fact, older studies showed that lower level chronic immune responses could actually be supportive of tumor growth. Prehn suggested that a moderate anti-tumor immune reaction is optimal for tumor growth and that many de novo tumors, rather than being inhibited, are probably dependent, at least early in their progression, upon eliciting an immune reaction (26). In keeping with this, there are many circumstances in which chronic inflammation plays a major role in carcinoma incidence and progression. Examples include colon cancer in ulcerative colitis, pancreatic cancer following chronic pancreatitis, hepatocellular carcinomas following chronic hepatitis, and gastric cancer in Helicobacter infections (31–33). This may reflect increased release of DNA-damaging oxidants (increasing mutation rate) and/or the availability of growth factors, cytokines, and angiogenic agents. This fits well with more recent data showing roles for antibodies and tumor-infiltrating immune cells as promoters of tumor growth and angiogenesis (30, 31, 33). As mentioned earlier, the effects of non-steroidal anti-inflammatory drugs (NSAID), such as aspirin and selective cyclooxygenase-2 (COX-2) inhibitors, in reducing the risk of cancer in humans (41–43) supports this concept. COX-2 is believed to promote cell survival and is involved in angiogenesis and inflammation. Other inflammatory mechanisms involving cytokines and the NFkappa-B pathway are also clearly implicated in carcinogenesis and tumor progression (48, 49).
Recent work by others has demonstrated that artificially-induced antibody-antigen reactions in premalignant or malignant tissues can set up a vicious cycle, in which the resulting chronic inflammation supports tumor progression, even while further boosting antibody responses (31, 33, 34). Our study shows how this mechanism could occur in a natural setting of human cancer. Notably, we also found that high levels of antibody administration in our experimental setting could instead inhibit tumor growth. This is also consistent with the original concept of a dualistic response of the tumor to the immune system. In this regard, it remains to be seen if very high natural levels of these antibodies might actually inhibit tumor growth and be protective in humans, and/or if such antibodies could eventually be isolated and harnessed for therapeutic purposes. Meanwhile, we have provided a novel explanation for the association of certain mammalian-derived foods with the incidence of various human carcinomas. To our knowledge, this is also the first example wherein a non-human dietary molecule becomes metabolically and covalently incorporated onto human cell surfaces, even in the face of an immune response against it.
Methods
Glycomic Analysis of Tumor Samples.
To examine all of the three major classes of sialylated glycans (N-glycans, O-glycans, and glycosphingolipids) for Neu5Gc content, tumor tissues were extracted using a previously described protocol with some modifications (50–52) (See SI Text for details). Samples were studied for sialic acid content by DMB-derivatization and HPLC analysis and the N-glycans profiled by MALDI-TOF Mass spectrometry (See SI Text).
Mice and Experimental Tumor Growth Assays.
Cmah−/− mice were generated as previously described (39). All mice were bred in a congenic C57BL/6 background and maintained in the University of California, San Diego vivarium according to Institutional Review Board guidelines for the care and use of laboratory animals. Mouse melanoma B16F1 cells were from ATCC and the mouse colon adenocarcinoma cell line MC-38 was a kind gift from J. Schlom (National Cancer Institute, Bethesda, MD). Both cell lines are syngeneic to C57BL/6 background and were cultured in DMEM with 10% FCS. All media and additives were from Life Technologies (Invitrogen), except for FCS, which was from HyClone. All cells were incubated at 37 °C with 5% CO2. Before use, cells were released by incubation in PBS with 2 mmol/L EDTA at 37 °C for 5 to 10 min, and washed in PBS with Ca2+, Mg2+ and glucose before suspending in the same buffer for i.v. injection. Mice were injected s.c. in the shaved flank with 0.5 × 106 cells. On day 5, anti-Neu5Gc antibodies from mouse or human were injected i.p. NS-398 (Cayman Chemical) was dissolved in dimethyl sulfoxide and injected i.p. into some mice as indicated in the text, which received 10 mg/kg of body weight every 3 days (total of three injections) immediately after injection of the tumor cells. The equivalent amount of dimethyl sulfoxide alone was injected i.p. into the other two groups of mice. Tumor growth was evaluated bi-weekly (mouse antibodies experiments) or daily (human antibodies experiment). At 2–3 weeks after injection (all mouse groups in each individual experiment were killed/harvested on the same day), tumors were removed from the flank, weighed, measured, cryoprotected with OCT, and frozen in a dry-ice/isopentane slurry. Tumor volume was calculated either by multiplying the longest dimension by the shortest dimension squared, or by the formula 0.5*x*y*z. Serial tumor sections were stained with hematoxylin and eosin. For the immunostaining experiments, biotinylated rat anti-mouse CD31,biotinylated rat anti-mouse Mac-1 and biotinylated rat anti mouse Gr-1 were from BDPharmingen. Binding of biotinylated antibodies was detected with Cy3-conjugated streptavidin (Jackson ImmunoResearch). FITC labeled anti-mouse IgG was from Jackson ImmunoResearch Laboratories. Quantitation of positive staining was performed using Adobe Photoshop (53).
ELISA Assays for Detection of Polyclonal Anti-Neu5Gc Antibodies in Mice.
Microtiter plate (Costar) wells were coated with 300 ng per well of Neu5Gcα-polyacrylamide (PAA), or Neu5Gcα2–6GalNAc-PAA (or with human or chimpanzee serum at 1:100 dilution - Neu5Gc negative and positive respectively) in 50 mM sodium carbonate-bicarbonate buffer, pH 9.5, at 4 °C overnight. After washing with TBS, pH 7.5, and blocking with TBST for 2 h at room temperature, triplicate wells were incubated with 1:50 dilutions of serum in TBST at room temperature for 4 h. Wells were washed five times with TBS and incubated with alkaline phosphatase-conjugated donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories), or anti-mouse IgM (Jackson ImmunoResearch Laboratories) each diluted in TBST at 1:5,000, at room temperature for 1.5 h. The IgG and IgM anti-Neu5Gc were quantitated by using a standard curve of normal mouse IgG coated to the wells under the same general conditions.
Preparation of RBC Membranes.
Chimpanzee RBC hemolysis was performed using 30 volumes of ice-cold 5 mM Tris·HCl/7 mM NaCl/1 mM EDTA, pH 8.0, followed by centrifugation at 20,000 × g for 15 min at 4 °C. Pelleted membranes were washed in the same buffer until white. Cmah−/− mice were immunized with 200 μl of these RBC membrane ghosts (200 μg in 100 μl PBS mixed with equal volume Freund's complete adjuvant) by intra-peritoneal injection. A booster injection using Freund's incomplete adjuvant with the same amount of immunogen was given two weeks later. Two weeks after the boost, serum was collected for analysis of anti-Neu5Gc responses. Positive sera were pooled and non-specific RBC reactivity was removed by repeated adsorption against human RBCs. Adsorption was performed using 100 μl packed washed human RBC incubated with pooled positive or negative control sera at 4 °C for 2 h and the RBCs subsequently removed by centrifugation. This procedure was repeated a total of three times, until the sera had no reactivity to human RBC, but continued reactivity with chimpanzee RBCs. The mouse anti-Neu5Gc IgG was quantitated using a standard curve of normal mouse IgG coated to the wells as described above.
Affinity Purification of Human Anti-Neu5Gc Antibodies.
Anti-Neu5Gc antibodies were purified from pooled human IgG on sequential affinity columns with immobilized human or chimpanzee sialoglycoproteins (rich with Neu5Ac or Neu5Gc, respectively) as previously described (24) and biotinylated using the EZ-Link Micro Sulfo-NHS-Biotinylation Kit (Pierce), according to the manufacturer instructions.
Flow Cytometry Analysis of MC-38 Cells.
Cells were detached using PBS containing 2 mM EDTA. After blocking with HBSS containing 1% BSA (HBSS/BSA), cells were incubated with mouse serum samples (diluted 1:50) or affinity purified anti-Neu5Gc antibodies (diluted 1:3) for 30 min on ice. After washing with HBSS/BSA, FITC-conjugated anti-mouse IgG or Cy5-streptavidin (Jackson ImmunoResearch Laboratories) were subsequently added and incubated for 30 min on ice. Cells were washed with HBSS/BSA and analyzed by flow cytometry.
Supplementary Material
Acknowledgments.
We thank Anup Datta and Natasha Naidu of the UCSD Glycotechnology Core for the glycan structural analyses. This work was supported by a postdoctoral fellowship from the STINT foundation (to M.H.), from the ISEF Foundation (to V.P.-K.) and NIH Grant R01CA38701 (to A.V.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0803943105/DCSupplemental.
References
- 1.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–5318. [PubMed] [Google Scholar]
- 2.Chou HH, et al. A mutation in human CMP-sialic acid hydroxylase occurred after the Homo-Pan divergence. Proc Natl Acad Sci USA. 1998;95:11751–11756. doi: 10.1073/pnas.95.20.11751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Irie A, Koyama S, Kozutsumi Y, Kawasaki T, Suzuki A. The molecular basis for the absence of N-glycolylneuraminic acid in humans. J Biol Chem. 1998;273:15866–15871. doi: 10.1074/jbc.273.25.15866. [DOI] [PubMed] [Google Scholar]
- 4.Higashi H, et al. Tumor-associated expression of glycosphingolipid Hanganutziu-Deicher antigen in human cancers. Gann. 1984;75:1025–1029. [PubMed] [Google Scholar]
- 5.Hirabayashi Y, Higashi H, Kato S, Taniguchi M, Matsumoto M. Occurrence of tumor-associated ganglioside antigens with Hanganutziu-Deicher antigenic activity on human melanomas. Jpn J Cancer Res. 1987;78:614–620. [PubMed] [Google Scholar]
- 6.Nakarai H, Saida T, Shibata Y, Irie RF, Kano K. Expression of heterophile, Paul-Bunnell and Hanganutziu-Deicher antigens on human melanoma cell lines. Int Arch Allergy Appl Immunol. 1987;83:160–166. doi: 10.1159/000234349. [DOI] [PubMed] [Google Scholar]
- 7.Ohashi Y, Sasabe T, Nishida T, Nishi Y, Higashi H. Hanganutziu-Deicher heterophile antigen in human retinoblastoma cells. Am J Ophthalmol. 1983;96:321–325. doi: 10.1016/s0002-9394(14)77822-5. [DOI] [PubMed] [Google Scholar]
- 8.Tangvoranuntakul P, et al. Human uptake and incorporation of an immunogenic nonhuman dietary sialic acid. Proc Natl Acad Sci USA. 2003;100:12045–12050. doi: 10.1073/pnas.2131556100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rose DP, Boyar AP, Wynder EL. International comparisons of mortality rates for cancer of the breast, ovary, prostate, and colon, and per capita food consumption. Cancer. 1986;58:2363–2371. doi: 10.1002/1097-0142(19861201)58:11<2363::aid-cncr2820581102>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 10.Giovannucci E, et al. A prospective study of dietary fat and risk of prostate cancer. J Natl Cancer Inst. 1993;85:1571–1579. doi: 10.1093/jnci/85.19.1571. [DOI] [PubMed] [Google Scholar]
- 11.Fraser GE. Associations between diet and cancer, ischemic heart disease, and all-cause mortality in non-Hispanic white California Seventh-day Adventists. Am J Clin Nutr. 1999;70:532S–538S. doi: 10.1093/ajcn/70.3.532s. [DOI] [PubMed] [Google Scholar]
- 12.Willett WC. Diet and cancer. Oncologist. 2000;5:393–404. doi: 10.1634/theoncologist.5-5-393. [DOI] [PubMed] [Google Scholar]
- 13.Tavani A, et al. La VC, Gallus S, Lagiou P, Trichopoulos D, Levi F, Negri E. Red meat intake and cancer risk: A study in Italy. Int J Cancer. 2000;86:425–428. doi: 10.1002/(sici)1097-0215(20000501)86:3<425::aid-ijc19>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 14.Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: Dose-response meta-analysis of epidemiological studies. Int J Cancer. 2002;98:241–256. doi: 10.1002/ijc.10126. [DOI] [PubMed] [Google Scholar]
- 15.Bosetti C, et al. Micelotta S, Dal Maso L, Talamini R, Montella M, Negri E, Conti E, Franceschi S, La Vecchia C. Food groups and risk of prostate cancer in Italy. Int J Cancer. 2004;110:424–428. doi: 10.1002/ijc.20142. [DOI] [PubMed] [Google Scholar]
- 16.Zhang J, Kesteloot H. Milk consumption in relation to incidence of prostate, breast, colon, and rectal cancers: Is there an independent effect? Nutr Cancer. 2005;53:65–72. doi: 10.1207/s15327914nc5301_8. [DOI] [PubMed] [Google Scholar]
- 17.Tseng M, Breslow RA, Graubard BI, Ziegler RG. Dairy, calcium, and vitamin D intakes and prostate cancer risk in the National Health and Nutrition Examination Epidemiologic Follow-up Study cohort. Am J Clin Nutr. 2005;81:1147–1154. doi: 10.1093/ajcn/81.5.1147. [DOI] [PubMed] [Google Scholar]
- 18.Bardor M, Nguyen DH, Diaz S, Varki A. Mechanism of uptake and incorporation of the non-human sialic acid N-glycolylneuraminic acid into human cells. J Biol Chem. 2005;280:4228–4237. doi: 10.1074/jbc.M412040200. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen DH, Tangvoranuntakul P, Varki A. Effects of natural human antibodies against a nonhuman sialic acid that metabolically incorporates into activated and malignant immune cells. J Immunol. 2005;175:228–236. doi: 10.4049/jimmunol.175.1.228. [DOI] [PubMed] [Google Scholar]
- 20.Yin J, et al. Hypoxic culture induces expression of sialin, a sialic acid transporter, and cancer-associated gangliosides containing non-human sialic acid on human cancer cells. Cancer Res. 2006;66:2937–2945. doi: 10.1158/0008-5472.CAN-05-2615. [DOI] [PubMed] [Google Scholar]
- 21.Merrick JM, Zadarlik K, Milgrom F. Characterization of the Hanganutziu-Deicher (serum-sickness) antigen as gangliosides containing N-glycolylneuraminic acid. Int Arch Allergy Appl Immunol. 1978;57:477–480. doi: 10.1159/000232140. [DOI] [PubMed] [Google Scholar]
- 22.Nowak JA, Jain NK, Stinson MW, Merrick JM. Interaction of bovine erythrocyte N-glycolylneuraminic acid-containing gangliosides and glycoproteins with a human Hanganutziu-Deicher serum. Mol Immunol. 1986;23:693–700. doi: 10.1016/0161-5890(86)90079-9. [DOI] [PubMed] [Google Scholar]
- 23.Morito T, Kano K, Milgrom F. Hanganutziu-Deicher antibodies in infectious mononucleosis and other diseases. J Immunol. 1982;129:2524–2528. [PubMed] [Google Scholar]
- 24.Padler-Karavani V, et al. Diversity in specificity, abundance, and composition of anti-Neu5Gc antibodies in normal humans: Potential implications for disease. Glycobiology. 2008;18:818–830. doi: 10.1093/glycob/cwn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prehn RT. The immune reaction as a stimulator of tumor growth. Science. 1972;176:170–171. doi: 10.1126/science.176.4031.170. [DOI] [PubMed] [Google Scholar]
- 26.Prehn RT, Prehn LM. The autoimmune nature of cancer. Cancer Res. 1987;47:927–932. [PubMed] [Google Scholar]
- 27.Prehn RT. Stimulatory effects of immune reactions upon the growths of untransplanted tumors. Cancer Res. 1994;54:908–914. [PubMed] [Google Scholar]
- 28.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 29.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 31.De Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. doi: 10.1038/nrc1782. [DOI] [PubMed] [Google Scholar]
- 32.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 33.Mantovani A. Cancer: Inflammation by remote control. Nature. 2005;435:752–753. doi: 10.1038/435752a. [DOI] [PubMed] [Google Scholar]
- 34.Tan TT, Coussens LM. Humoral immunity, inflammation and cancer. Curr Opin Immunol. 2007;19:209–216. doi: 10.1016/j.coi.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Bui JD, Schreiber RD. Cancer immunosurveillance, immunoediting and inflammation: Independent or interdependent processes? Curr Opin Immunol. 2007;19:203–208. doi: 10.1016/j.coi.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 36.de Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Kato A, Higashi H, Kato S, Naiki M. Quantitative determination of N-glycolylneuraminic acid expression in human cancerous tissues and avian lymphoma cell lines as a tumor-associated sialic acid by gas chromatography-mass spectrometry. Cancer Res. 1991;51:1242–1246. [PubMed] [Google Scholar]
- 38.Devine PL, et al. The breast tumor-associated epitope defined by monoclonal antibody 3E1.2 is an O-linked mucin carbohydrate containing N-glycolylneuraminic acid. Cancer Res. 1991;51:5826–5836. [PubMed] [Google Scholar]
- 39.Hedlund M, et al. N-glycolylneuraminic acid deficiency in mice: implications for human biology and evolution. Mol Cell Biol. 2007;27:4340–4346. doi: 10.1128/MCB.00379-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muchmore EA, Diaz S, Varki A. A structural difference between the cell surfaces of humans and the great apes. Am J Phys Anthropol. 1998;107:187–198. doi: 10.1002/(SICI)1096-8644(199810)107:2<187::AID-AJPA5>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 41.Gupta RA, Dubois RN. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nat Rev Cancer. 2001;1:11–21. doi: 10.1038/35094017. [DOI] [PubMed] [Google Scholar]
- 42.Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: Rationale and promise. Cancer Cell. 2003;4:431–436. doi: 10.1016/s1535-6108(03)00310-6. [DOI] [PubMed] [Google Scholar]
- 43.Mann JR, Backlund MG, DuBois RN. Mechanisms of disease: Inflammatory mediators and cancer prevention. Nat Clin Pract Oncol. 2005;2:202–210. doi: 10.1038/ncponc0140. [DOI] [PubMed] [Google Scholar]
- 44.Key TJ, et al. Mortality in vegetarians and nonvegetarians: Detailed findings from a collaborative analysis of 5 prospective studies. Am J Clin Nutr. 1999;70:516S–524S. doi: 10.1093/ajcn/70.3.516s. [DOI] [PubMed] [Google Scholar]
- 45.Varki A. A chimpanzee genome project is a biomedical imperative. Genome Res. 2000;10:1065–1070. doi: 10.1101/gr.10.8.1065. [DOI] [PubMed] [Google Scholar]
- 46.Pardoll DM. Spinning molecular immunology into successful immunotherapy. Nat Rev Immunol. 2002;2:227–238. doi: 10.1038/nri774. [DOI] [PubMed] [Google Scholar]
- 47.Finn OJ. Cancer immunology. N Engl J Med. 2008;358:2704–2715. doi: 10.1056/NEJMra072739. [DOI] [PubMed] [Google Scholar]
- 48.Lin WW, Karin M. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Naugler WE, Karin M. The wolf in sheep's clothing: The role of interleukin-6 in immunity, inflammation and cancer. Trends Mol Med. 2008;14:109–119. doi: 10.1016/j.molmed.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 50.Manzi AE, et al. Exploring the glycan repertoire of genetically modified mice by isolation and profiling of the major glycan classes and nano-NMR analysis of glycan mixtures. Glycobiology. 2000;10:669–689. doi: 10.1093/glycob/10.7.669. [DOI] [PubMed] [Google Scholar]
- 51.Norgard-Sumnicht K, Bai XM, Esko JD, Varki A, Manzi AE. Exploring the outcome of genetic modifications of glycosylation in cultured cell lines by concurrent isolation of the major classes of vertebrate glycans. Glycobiology. 2000;10:691–700. doi: 10.1093/glycob/10.7.691. [DOI] [PubMed] [Google Scholar]
- 52.Sonnenburg JL, Van Halbeek H, Varki A. Characterization of the acid stability of glycosidically linked neuraminic acid—Use in detecting de-N-acetyl-gangliosides in human melanoma. J Biol Chem. 2002;277:17502–17510. doi: 10.1074/jbc.M110867200. [DOI] [PubMed] [Google Scholar]
- 53.Lehr HA, Mankoff DA, Corwin D, Santeusanio G, Gown AM. Application of photoshop-based image analysis to quantification of hormone receptor expression in breast cancer. J Histochem Cytochem. 1997;45:1559–1565. doi: 10.1177/002215549704501112. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.