Abstract
Objective
To determine the association between fatigue and survival over ten years in a population of community-dwelling older primary care patients.
Design
Prospective cohort study.
Setting
Medicare health maintenance organization and Veterans Affairs primary care programs.
Participants
Older primary care patients (n=492).
Measurements
Fatigue, operationalized as feeling tired most of the time, was assessed at baseline. Mortality was ascertained from the National Death Index. Covariates included demographics, comorbidity, cognitive function, depressive symptoms, body mass index, self-rated health, functional status, and gait speed.
Results
Mortality rates for older adults with and without fatigue were 59% (123/210) versus 38% (106/282) at 10 years (p<0.001). After adjustment for multiple potential confounders, participants who were tired at baseline had a greater risk of death than those who were not (hazard ratio 1.44, 95% confidence interval 1.08-1.93).
Conclusions
A single simple question “Do you feel tired most of the time?” identifies older adults with a higher risk of mortality. Further research is needed to identify and characterize the underlying mechanisms of fatigue, to develop and test specific treatments, and to determine if improvement leads to decreased morbidity and mortality.
Keywords: fatigue, mortality, cohort studies, aged
Introduction
Fatigue, or persistent unexplained tiredness, may be a novel and important geriatric syndrome because it has serious consequences and might reflect underlying and potentially modifiable pathophysiology. Among older adults, fatigue is common and associated with functional deficits both cross-sectionally and longitudinally,1-4 but less is known about the relationship between fatigue and survival. Fatigue symptoms are associated with mortality in cardiovascular disease,5-7, stroke,8 and breast cancer.9 In a general population of older adults, Avlund has reported that tiredness when performing activities of daily living is associated with subsequent mortality.10
The purpose of this study is to determine the association between fatigue and survival over ten years in a population of community-dwelling older primary care patients. We operationalized fatigue as tiredness, specifically feeling tired most of the time in the past month. Tiredness is one of the most commonly used synonyms for fatigue in published fatigue scales,4, 11-13 and we did not have data available directly assessing “fatigue.”
Methods
Overview
Subjects were recruited from two primary care clinics (a Medicare Health Maintenance Organization and a Veterans Affairs clinic) in 1996 and followed for 36 months. The purpose of the original study was to determine the feasibility and effectiveness of physical performance measures as predictors of health and functional outcomes in the primary care setting. The present study uses baseline data and mortality data from a national death index14 ascertained up to January 1, 2007. The study was approved by the relevant Institutional Review Boards. Study methods, described in detail elsewhere,15 are summarized below.
Subjects
Community-dwelling primary care patients aged 65 years or older were eligible if they were cognitively intact (Mini Mental State Examination (MMSE)16 score ≥ 24) or mildly impaired (MMSE 16-23) with a caregiver, were able to walk 4 meters, and had a gait speed between 0.2 and 1.3 meters per second (m/s). Participants who used assistive devices were included.
Assessment of Tiredness
As part of a symptom assessment performed at each visit, participants were asked if, during the past month, they had been “feeling tired most of the time.” We use the baseline tiredness assessment for these analyses.
Measures
In addition to tiredness, participants underwent assessment of demographic characteristics, self-rated health,11 cognition,16 and self-reported physician-diagnosed chronic conditions.17 Sleep problems and chronic pain were assessed with the question “Has a doctor ever told you that you have (a sleep problem/chronic pain)?” We also considered alternate definitions of sleep difficulty and pain, based on the question “In the past month, have you had [the symptom]?” Depressive symptoms were assessed with the Geriatric Depression Scale (GDS).18 Because fatigue is a symptom of depression, we also created a scale using four items assessing mood (life is empty, downhearted and blue, good spirits, happy most of the time). Body Mass Index (BMI) was calculated from height and weight from the medical record. Functional status was measured with the 100 point physical function index (PFI) of the Medical Outcomes Study Short Form (SF-36)11 and the number of 16 basic and instrumental activities of daily living (ADL) from the National Health Interview Survey (NHIS) performed without difficulty.19 Usual gait speed was assessed over a four-meter course. Inter-rater and test-retest reliability for the measures used in this study was found to be excellent with intra-class correlations generally over 0.9.15 Date of death was determined using the Social Security National Death Index,14 which confirms all deaths through a family member or death certificate.
Statistical analysis
Mortality rates in those with and without tiredness were compared. Kaplan-Meier product-limit survival curves were used to depict survival graphically and Cox proportional hazards models were used to estimate hazard ratios (HR), their 95% confidence intervals (CI) and p-values to compare the rates of death of those with and without tiredness. The proportional hazards assumption was checked by including the interaction of time and tiredness in the model; these interaction terms were not significant. We present 4 models: 1) unadjusted; 2) adjusted for demographics; 3) adjusted for demographics, chronic diseases, BMI, cognition, depressive symptoms, sleep problems, and chronic pain; and 4) adjusted for the factors in model 3, self-rated health, functional status, and physical performance. In addition, we examine the adjusted associations of fatigue and survival in several key subgroups of older adults, based on age, gender, race, number of comorbid conditions, functional status, and gait speed. SAS® version 8.2 (SAS Institute, Cary, North Carolina) was used for all analyses.
Results
Of the 572 individuals screened, 492 (86%) entered the study and were followed for up to ten years for mortality. The study population had a mean age of 74 years and 44.4% were female (Table 1, first column). Men were overrepresented in this study because of recruitment from a Veterans Affairs clinic. At baseline, 210 participants (43%) reported feeling tired most of the time. Participants who reported tiredness were more likely to be female and white (Table 1). Tiredness was associated with greater prevalence of specific conditions, more concurrent conditions, more depressive symptoms, worse functional status, and worse physical performance.
Table 1.
Participant Characteristics by Baseline Tiredness*
| Tired most of the time in past month | ||||
|---|---|---|---|---|
| Characteristics | Total
(n=492) |
Yes
(n=210) |
No
(n=282) |
p-value |
| Age, years | 74.1 ± 5.7 | 74.4 ± 5.6 | 73.8 ± 5.8 | .36 |
| Female | 216 (44) | 105 (50) | 111 (39) | .02 |
| White | 394 (80) | 177 (84) | 217 (77) | .04 |
| High school or greater education | 324 (66) | 138 (66) | 186 (66) | .96 |
| Number of chronic conditions (of 8)† | 2.4 ± 1.4 | 3.0 ± 1.3 | 2.0 ± 1.2 | <.001 |
| Cardiovascular | 111 (23) | 55 (26) | 56 (20) | 0.10 |
| Neurological | 52 (11) | 29 (14) | 23 (8) | 0.04 |
| Musculoskeletal | 349 (71) | 166 (79) | 184 (65) | <0.001 |
| Pulmonary | 118 (24) | 69 (33) | 49 (17) | <0.001 |
| Diabetes | 85 (17) | 51 (24) | 34 (12) | <0.001 |
| Cancer | 113 (23) | 54 (26) | 59 (21) | 0.21 |
| Visual | 263 (53) | 129 (61) | 134 (48) | 0.002 |
| General | 103 (21) | 69 (33) | 34 (12) | <0.001 |
| Cognitive function (MMSE‡) | 27.5 ± 2.3 | 27.5 ± 2.3 | 27.4 ± 2.4 | .64 |
| Depressive symptoms (GDS§) | 2.3 ± 2.8 | 3.6 ± 3.1 | 1.4 ± 2.0 | <0.001 |
| Depressive symptoms (mood items§) | 0.3 ± 0.8 | 0.5 ± 0.9 | 0.2 ± 0.6 | <0.001 |
| Sleep problems | 30 (6) | 21 (10) | 9 (3) | 0.002 |
| Chronic Pain | 38 (8) | 26 (12) | 12 (4) | <0.001 |
| Body mass index, kilograms/meters2 | 27.5 ± 5.1 | 28.0 ± 5.5 | 27.2 ± 4.8 | .10 |
| Self-rated health fair or poor | 108 (22) | 72 (34) | 36 (13) | <0.001 |
| NHIS ADL‖ | 14 ± 2 | 13 ± 2.5 | 15 ± 1.6 | <0.001 |
| SF-36 PFI# | 64 ± 30 | 50 ± 28 | 74 ± 26 | <0.001 |
| Gait speed, meters/second | .88 ± .24 | .82 ± .24 | .92 ± .23 | <0.001 |
Values represent N (%) for dichotomous variables and mean ± standard deviation for continuous variables.
Chronic condition categories include: cardiovascular (angina, heart failure, or heart attack), neurological (stroke or Parkinson's disease), pulmonary (lung disease, emphysema, asthma, or bronchitis), musculoskeletal (arthritis, osteoporosis, broken bone, amputation, or joint replacement), diabetes, cancer, visual (cataracts or glaucoma), and general (depression/anxiety/emotional problem, sleep problem, or chronic pain).
MMSE: Mini-mental State Exam, range 0-30 with higher scores representing better cognition
GDS: Geriatric Depression Score, range 0-15 with higher scores representing more depressive symptoms; we also present a 4-item scale using only mood-items (life is empty, downhearted and blue, good spirits, happy most of the time) with a range of 0-4
NHIS ADL: National Health Interview Survey Activities of Daily Living, range 0-16 with higher scores representing better function
SF-36 PFI: Medical Outcomes Survey Physical Function Index, range 0-100 with higher scores representing better function
During the ten years of follow-up, 229 participants (47%) died. Participants who reported tiredness were more likely to die than those without tiredness throughout the ten-year follow-up (see Figure 1 for survival curves). Mortality rates for those with and without tiredness were 10% versus 4% at 2 years (p=0.01), 28% versus 15% at 5 years (p<0.001), and 59% versus 38% at 10 years (p<0.001). This difference in survival, which continued to increase throughout the 10-year follow-up, persisted despite adjustment for demographics, chronic conditions, BMI, cognition, depressive symptoms, sleep problems, chronic pain, functional status, and physical performance (Table 2). The magnitude of the association of fatigue with mortality (HR 1.44) was similar to that of diabetes (HR 1.42) and heart disease (1.57). This association was consistent across key subgroups based on age, gender, race, comorbidity and gait speed (although not always statistically significant), with the exception of subjects with no disability and non-white race (Table 2). A formal statistical test of an interaction between functional status and fatigue was not significant (p=0.18). Fatigue tended to be more strongly associated with mortality in non-whites than in whites, although the interaction between race and fatigue was not statistically significant (p=0.10). Use of alternate definitions of sleep problems and pain, based on simple report of the symptoms, resulted in a higher prevalence of these symptoms, but did not substantially alter the results of the multivariable models.
Figure 1.
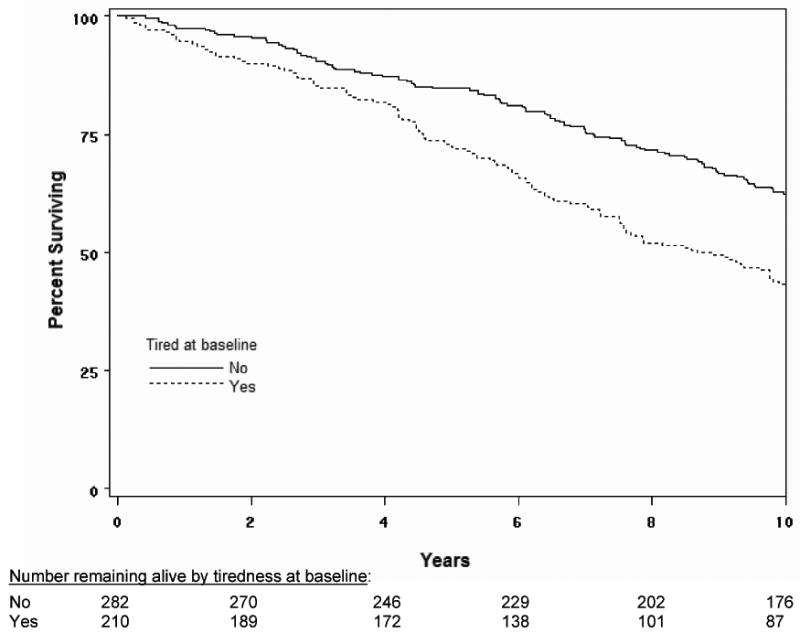
Ten-year survival based on Kaplan-Meier survival curves among older adults with and without tiredness at baseline. P<0.001 for comparison across groups.
Table 2.
Effect of Baseline Tiredness on 10-Year Survival in the Full Cohort and Key Subgroups
| Full Cohort Models | Hazard Ratio
(95% CI) |
p-value | |
|---|---|---|---|
| 1 Unadjusted | 1.86 (1.44-2.41) | <0.001 | |
| 2 Adjusted for age, gender, race, education | 1.94 (1.49-2.53) | <0.001 | |
| 3 Model 2 plus BMI, MMSE, cardiovascular disease, neurological disease, diabetes, cancer, musculoskeletal disease, pulmonary disease, ophthalmologic disease, depressive symptoms, sleep problems, chronic pain | 1.69 (1.27-2.25) | <0.001 | |
| 4 Model 3 plus self-rated health, SF-36 PFI, NIH ADL, and gait speed* | 1.44 (1.08-1.93) | 0.01 | |
| Fully Adjusted Models for Key Subgroups | |||
| Age: | < 75 years (n=212) | 1.53 (0.99-2.39) | 0.06 |
| ≥ 75 years (n=280) | 1.51 (1.01-2.27) | 0.05 | |
| Gender: | Men (n=276) | 1.60 (1.10-2.34) | 0.01 |
| Women (n=216) | 1.52 (0.94-2.48) | 0.09 | |
| Race: | White (n=394) | 1.39 (1.01-1.90) | 0.04 |
| Non-white (n=98) | 2.91 (1.33-6.42) | 0.008 | |
| Comorbid | ≤ 2 (n=275) | 1.48 (0.93-2.36) | 0.10 |
| conditions: | > 2 (n=217) | 1.38 (0.93-2.04) | 0.10 |
| Functional | No difficulty with ADLs or IADLs (n=197) | 0.85 (0.47-1.54) | 0.58 |
| Status: | Difficulty with ≥ 1 ADL or IADL (n=295) | 1.85 (1.28-2.66) | 0.001 |
| Gait speed: | < 1 meter/second (n=337) | 1.39 (1.00-1.94) | 0.05 |
| ≥ 1 meter/second (n=155) | 1.63 (0.73-3.63) | 0.24 | |
BMI: Body mass index; MMSE: Mini-mental State Exam; NHIS ADL: National Health Interview Survey Activities of Daily Living; SF-36 PFI: Medical Outcomes Survey Physical Function Index; ADL: Instrumental Activities of Daily Living; IADL: Instrumental Activities of Daily Living
The other significant predictors of mortality (and HRs per unit increase) in the fully adjusted model were age (1.05), female gender (0.69), white race (1.79), BMI (0.95), cardiovascular disease (1.57), diabetes (1.42), lung disease (1.47), chronic pain (1.77), gait speed (0.39), and the SF-36 PFI (0.99).
Discussion
Fatigue, defined as self-report of feeling tired most of the time over the prior month, is associated with increased mortality over 10 years. While tiredness was associated with other factors that affect survival, such as chronic conditions and other indicators of health and function, the association of fatigue with mortality persisted despite adjustment for these conditions. The magnitude of this association was consistent across key subgroups, with the exception of the subset of participants with the best functional status.
These findings, together with Schultz-Larson's examination of task-specific tiredness,10 indicate that fatigue or tiredness is a robust independent predictor of mortality. Subjective fatigue, alone without the other proposed criteria for the frailty syndrome,20 is a risk factor for multiple adverse outcomes among older adults. Unfortunately, we know little about the pathophysiology of fatigue in older adults. While the current study cannot address potential underlying mechanisms of the effect of fatigue on survival, the association despite adjustment for a variety of markers of mental and physical health suggests that it may be the symptomatic expression of a more general pathology. Fatigue could be the symptomatic presentation of subclinical disease, increased inflammation, or increased work in maintaining homeostasis.20, 21 A better understanding of the pathophysiologic origins of fatigue is a necessary first step in the development of new preventive and therapeutic strategies.
While the overall association of fatigue with survival is fairly robust, self-report of tiredness was not associated with mortality among older persons who reported no functional limitations, with a point estimate close to 1. Although there was not a statistical difference in the effect of fatigue on survival between those with and without functional limitations, the difference in the magnitude and direction of the point estimates suggests that tiredness may be interpreted differently by older adults with different functional abilities. For example, the functionally independent may be more active and experience tiredness due to a lack of sufficient rest, while those who are more dependent may experience tiredness despite rest. Similarly, our results suggest that fatigue is a greater risk factor for mortality among non-whites compared to whites. The subjective experience of fatigue or its physiologic associations may differ by race. Further research is needed to disentangle the relationships among function, fatigue, race, and mortality in older adults.
The strengths of this study include the clinically relevant patient sample drawn from primary care clinics, the availability of data on key variables, and the long follow up. The limitations include incomplete characterization of fatigue and the lack of physiological measures. Tiredness, although commonly used as a synonym for fatigue, may not encompass all aspects of fatigue (e.g. weakness, sleepiness, or cognitive fatigue). In addition, some participants who report tiredness might not consider themselves fatigued. We did not assess fatigue severity, and thus cannot assess a dose-response relationship between fatigue and function. We did not have measures of physiologic abnormalities, such as anemia or thyroid function, that might help explain our findings. We did not have data on some predictors of mortality, such as smoking, and therefore may not have adjusted for all potential confounders.
This study demonstrates that a single simple question “Do you feel tired most of the time?” identifies older adults with a higher risk of mortality. Further research is needed to identify and characterize the underlying mechanisms of fatigue, to develop and test specific treatments, and to determine if improvement leads to decreased morbidity and mortality.
Acknowledgments
| Financial/Personal Conflict Elements | S Hardy | S Studenski | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Employment or Affiliation | X | X | ||
| Grants/Funds | X | X | ||
| Honoraria | X | X | ||
| Speaker Forum | X | X | ||
| Consultant | X | X | ||
| Stocks | X | X | ||
| Royalties | X | X | ||
| Expert Testimony | X | X | ||
| Board Member | X | X | ||
| Patents | X | X | ||
| Personal Relationship | X | X | ||
Funding Sources: Merck Research Laboratories; Pittsburgh Claude D. Pepper Older Americans Independence Center (NIA P30 AG-024827); National Institute on Aging (K07 AG023641); and the Hartford Foundation's Pittsburgh Center of Excellence in Geriatric Medicine.
Role of the funding source: Merck employees participated in the design and analysis of the original cohort study. The authors had full access to the data, performed the analyses for this study, drafted the manuscript, and made the decision to submit the manuscript for publication.
Footnotes
COI Details:
Susan Hardy: Recipient of a Pfizer/AGS Foundation for Health in Aging Junior Faculty Scholars Grant, 7/04 to 6/06.
Stephanie Studenski: Recipient of grants from Ortho Biotech (Responsiveness and meaningful change in two common physical performance measures of mobility, 9/04-4/05) and Lilly Pharmaceuticals (Development of a Clinical Global Impressions of Frailty Scale, 9/02-6/07). Consultant for Asubio, Pfizer, Glaxo Smith Kline, and Merck.
Author Contributions: Susan E. Hardy–study concept and design, analysis and interpretation of data, drafting of manuscript; Stephanie A. Studenski– study concept and design, collection and assembly of data, analysis and interpretation of data, drafting of manuscript.
References
- 1.Liao S, Ferrell BA. Fatigue in an older population. J Am Geriatr Soc. 2000;48:426–430. doi: 10.1111/j.1532-5415.2000.tb04702.x. [DOI] [PubMed] [Google Scholar]
- 2.Vestergaard S, Nayfield S, Patel K, Eldadah B, Cesari M, Ferrucci L, Ceresini G, Guralnik J. Fatigue in a representative population of older persons and its association with functional impairment, functional limitation and disability. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/gln017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy SE, Studenski SA. Fatigue and function over three years among older adults. J Gerontol A Biol Sci Med Sci. doi: 10.1093/gerona/63.12.1389. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avlund K, Damsgaard MT, Sakari-Rantala R, Laukkanen P, Schroll M. Tiredness in daily activities among nondisabled old people as determinant of onset of disability. J Clin Epidemiol. 2002;55:965–973. doi: 10.1016/s0895-4356(02)00463-8. [DOI] [PubMed] [Google Scholar]
- 5.Prescott E, Holst C, Gronbaek M, Schnohr P, Jensen G, Barefoot J. Vital exhaustion as a risk factor for ischaemic heart disease and all-cause mortality in a community sample. A prospective study of 4084 men and 5479 women in the Copenhagen City Heart Study. Int J Epidemiol. 2003;32:990–997. doi: 10.1093/ije/dyg235. [DOI] [PubMed] [Google Scholar]
- 6.Cole SR, Kawachi I, Sesso HD, Paffenbarger RS, Lee IM. Sense of exhaustion and coronary heart disease among college alumni. Am J Cardiol. 1999;84:1401–1405. doi: 10.1016/s0002-9149(99)00585-8. [DOI] [PubMed] [Google Scholar]
- 7.Appels A, Mulder P. Excess fatigue as a precursor of myocardial infarction. Eur Heart J. 1988;9:758–764. doi: 10.1093/eurheartj/9.7.758. [DOI] [PubMed] [Google Scholar]
- 8.Glader EL, Stegmayr B, Asplund K. Poststroke fatigue: a 2-year follow-up study of stroke patients in Sweden. Stroke. 2002;33:1327–1333. doi: 10.1161/01.str.0000014248.28711.d6. [DOI] [PubMed] [Google Scholar]
- 9.Groenvold M, Petersen MA, Idler E, Bjorner JB, Fayers PM, Mouridsen HT. Psychological distress and fatigue predicted recurrence and survival in primary breast cancer patients. Breast Cancer Res Treat. 2007;105:209–219. doi: 10.1007/s10549-006-9447-x. [DOI] [PubMed] [Google Scholar]
- 10.Schultz-Larsen K, Avlund K. Tiredness in daily activities: a subjective measure for the identification of frailty among non-disabled community-living older adults. Arch Gerontol Geriatr. 2007;44:83–93. doi: 10.1016/j.archger.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 11.Ware JJ, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–483. [PubMed] [Google Scholar]
- 12.Okuyama T, Akechi T, Kugaya A, Okamura H, Shima Y, Maruguchi M, Hosaka T, Uchitomi Y. Development and validation of the cancer fatigue scale: a brief, three-dimensional, self-rating scale for assessment of fatigue in cancer patients. J Pain Symptom Manage. 2000;19:5–15. doi: 10.1016/s0885-3924(99)00138-4. [DOI] [PubMed] [Google Scholar]
- 13.Piper BF, Dibble SL, Dodd MJ, Weiss MC, Slaughter RE, Paul SM. The revised Piper Fatigue Scale: psychometric evaluation in women with breast cancer. Oncol Nurs Forum. 1998;25:677–684. [PubMed] [Google Scholar]
- 14.Social Security Death Index. [July 31, 2006]; http://ssdi.genealogy.rootsweb.com/cgi-bin/ssdi.cgi.
- 15.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, Fox M, Guralnik JM. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 16.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state:” a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 17.Rigler SK, Studenski S, Wallace D, Reker DM, Duncan PW. Co-morbidity adjustment for functional outcomes in community-dwelling older adults. Clin Rehabil. 2002;16:420–428. doi: 10.1191/0269215502cr515oa. [DOI] [PubMed] [Google Scholar]
- 18.Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS). Recent evidence and development of a shorter version. In: Brink TL, editor. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press, Inc.; 1986. pp. 165–173. [Google Scholar]
- 19.Fitti JE, Kovar MG. Vital & Health Statistics, Series 2, No 2. Hyatsville, MD: DHHS; 1987. The supplement on aging to the 1984 National Health Interview Survey; pp. 1–115. Pub No. PHS 87-1323. [PubMed] [Google Scholar]
- 20.Walston J, Hadley EC, Ferrucci L, Guralnik JM, Newman AB, Studenski SA, Ershler WB, Harris T, Fried LP. Research agenda for frailty in older adults: toward a better understanding of physiology and etiology: summary from the American Geriatrics Society/National Institute on Aging Research Conference on Frailty in Older Adults. J Am Geriatr Soc. 2006;54:991–1001. doi: 10.1111/j.1532-5415.2006.00745.x. [DOI] [PubMed] [Google Scholar]
- 21.Ruggiero C, Ferrucci L. The endeavor of high maintenance homeostasis: resting metabolic rate and the legacy of longevity. J Gerontol A Biol Sci Med Sci. 2006;61:466–471. doi: 10.1093/gerona/61.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]


