Abstract
We extracted six Hong Kong brown seaweed species with hot water for their antiviral properties. The cytotoxicity and antiviral activity of these extracts were tested by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenlytetrezolium bromide] method, cytopathic effect reduction assay, and plaque reduction assay. The antiviral effect was further determined by flow cytometric analysis. The results showed that most of these extracts inhibited the propagation of herpes simplex virus types 1 and 2 (HSV-1 and HSV-2) standard strains with very low cytotoxicity to the host cells. The extracts of Hydroclathrus clathratus and Lobophora variegata showed more potential anti-HSV activities than the extracts of the other four seaweeds. They also had moderate anti-respiratory syncytial virus (RSV) activities but could not inhibit influenza A virus. Hydroclathrus clathratus was further extracted by diluted acid and alkali and the antiviral effects of the extracts were also detected. The result showed that the hot water extract contained the main carbohydrate components that exhibited the antiviral activities against various strains of HSV, including the acyclovir-resistant strain. HI-3, a compound fractionated from this hot water extract, showed a dose-dependent anti-HSV activity in flow cytometric analysis and plaque reduction assay.
Keywords: Hong Kong seaweed, Hydroclathrus clathratus, Antiviral activity, Herpes simplex virus (HSV), Flow cytometry
INTRODUCTION
Viruses are responsible for many serious illnesses. Respiratory syncytial virus (RSV), for example, is a major cause of lower respiratory tract disease especially in infants, children and the elderly (Scott et al., 2007). Despite much effort, it has not yet been possible to develop an effective vaccine against RSV and therapy for respiratory tract disease caused by RSV is still not available (Kneyber et al., 2000; Becker, 2007). Even if such vaccines exist, problems may continue to arise since virus changes rapidly, so vaccine developed for it must be reformulated frequently. The nucleoside analog ribavirin is used currently in antiviral therapy against RSV and influenza virus, but the efficacy, value and safety of ribavirin remain to be clarified (de Clercq, 1996). Herpes virus is a kind of ancient virus that causes different types of infections. Approximately 80% of the adult population worldwide are infected with herpes simplex virus (HSV) type 1 (HSV-1) and approximately 20% of them are also infected with HSV type 2 (HSV-2) (Chutkowski et al., 2002). Until now, a number of nucleoside analogues, especially the guanosine analogue acyclovir, have been developed as antiherpetic agents (Alché et al., 2002). The therapeutic limitation of these nucleoside analogues is that drug resistant strains develop readily through mutations in viral genes for thymidine kinase and/or polymerase. Therefore, the continuous search for new compounds as antiviral agents is urgently needed (Ammendolia et al., 2007).
New types of antiviral agents from natural sources, especially those that have high efficacy on resistant mutant viral strains and low toxicity to host, are considered to be most promising. Products from marine organisms show many interesting activities. Their constituents are more novel than those of many land plants. Seaweeds have long been recognized as rich and valuable natural resources of bioactive compounds because of their various biological properties (Mayer and Lehmann, 2000). The water-soluble extracts of seaweeds have been shown to exhibit antiviral activity against a wide spectrum of viruses (Witvrouw and de Clercq, 1997). There are more than 200 species of seaweeds in Hong Kong coastal waters (Ang, 2005), but research on their antiviral activity is very limited (Zhu et al., 2003). In this study, crude water extracts of six species of seaweeds from Hong Kong coastal waters were examined for their cellular toxicity and antiherpetic activity. Their inhibitory effects on RSV and influenza A virus were also tested. Aqueous extracts by ethanol, acid and alkali extracts of one of these species, Hydroclathrus clathratus (C. Agardh) M.A. Howe, were further evaluated for their antiviral activities as well.
MATERIALS AND METHODS
Seaweed samples
Six species of brown seaweeds, including Colpomenia sinuosa (Mertens ex Roth), Dictyota dichotoma (Hudson) J.V. Lamouroux, Hydroclathrus clathratus, Lobophora variegata (Lamouroux) Womersley ex Oliveira, Padina australis Holmes and Sargassum hemiphyllum (Turner) C. Agardh, were collected from Hong Kong coastal waters in March, April and May, 2002. Voucher specimens of these materials were deposited for reference in the Marine Science Laboratory, the Chinese University of Hong Kong. These seaweeds were stored at −20 °C until use.
Viruses, cells and positive control compounds
HSV-1, HSV-2 clinical strains and influenza virus A H1N1 strain were obtained from the Department of Microbiology, Prince of Wales Hospital, the Chinese University of Hong Kong. HSV-1 15577 strain (standard strain), HSV-2 8702 strain (standard strain), HSV-1 DM2.1 strain (acyclovir-resistant strain with thymidine kinase deficiency) and RSV Long strain were kindly provided by Dr. Spence H.S. Lee (Department of Microbiology and Immunology, Dalhousie University, Halifax, N.S., Canada). The viral titer was tested by the cytopathic end-point assay (Burleson et al., 1992) and expressed as 50% tissue culture infective dose (TCID50).
Vero cell (ATCC CCL-81, African green monkey kidney cell line), HEp-2 cell (ATCC CCL-23, human larynx epidermoid carcinoma cell line) and MDCK cell (ATCC CCL-34, Madin Darby canine kidney cell line) were used as the hosts for HSV, RSV and influenza A virus, respectively. All of the cells were cultured in growth medium (GM), the Eagle’s minimum Essential medium (EMEM, GIBCO™, USA) supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS, GIBCO™, USA) and incubated at 37 °C in 95% humidified atmosphere and 5% (v/v) CO2 incubator. Maintenance medium (MM) was the same EMEM but containing 1% (v/v) fetal bovine serum (FBS).
In antiviral assay, acyclovir (ACV, the Wellcome Foundation Ltd., England) and ribavirin, two clinically used medicines for treating viral infections, and dextran sulfate (DS, MW 10 000, Sigma, USA), a sulfated polysaccharide with well-known antiherpetic properties isolated from seaweed, were used as positive control compounds.
Extraction
The seaweeds were thoroughly washed with running water to remove salt and all visible epiphytes. They were put out to air-dry for 24 h and then minced with a blender separately. The extraction was carried out in boiling water for 2 h. After centrifugation and filtration, the supernatant was condensed and then lyophilized to obtain brown or dark brown crude water extracts.
The seaweed (Hydroclathrus clathratus) that showed the highest antiviral activity was chosen for further treatment. The crude water extract was precipitated by ethanol at the final concentration of 30% and 70% (v/v). The residue of water extract was divided into two parts and extracted by 0.1 mol/L of hydrochloric acid and 1 mol/L of sodium hydroxide, respectively, at 4 °C and kept for overnight. After centrifugation, the supernatants were adjusted to about pH 7.0 and precipitated by ethanol with the final concentration of 80% (v/v). All precipitates were dissolved in distilled water, dialyzed and lyophilized, and four components were obtained. The antiviral activities of these compounds against HSV were then tested separately.
Cellular toxicity test
The morphological changes of cells induced by test samples were observed under inverted microscope (de Clercq, 1985). Confluent cell monolayers were treated with the extracts in MM at a serial twofold dilution or only with MM (as cell control) at 37 °C for 3 d. A disruption of the cell monolayer was observed using an inverted microscope (Nikon CMM 214, Japan). The maximal non-cytotoxic concentration (MNCC) of the extract was determined by comparing the shape of the treated cells with that of the untreated control.
The cytotoxicity of seaweed extracts was tested by MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenlytetrezolium bromide] assay as previously described (Wang et al., 2007a). After the cells were treated with extract solutions at various concentrations for 3 d, MTT (Sigma) solution [5 mg/ml in phosphate buffered saline (PBS)] was added (12 μl/well) and incubated for additional 4 h. The old medium and redundant MTT were replaced by acid-isopropanol (0.04 mol/L HCl in isopropanol, 150 μl per well) to dissolve the dark blue crystals. The absorbance of the solution was then read at 570 nm with a reference wavelength of 630 nm. The 50% cytotoxicity concentration (CC50) was estimated from the plots graphically.
Cytopathic effect reduction assay
The antiviral activities of the six samples were first tested by cytopathic effect (CPE) reduction assay (Serkedjieva and Ivancheva, 1998). In brief, a serial twofold dilution of extracts in MM (50 μl per well) and equal volume of virus suspension (1 000 TCID50) were added to the confluent cell monolayers in a 96-well plate (IWAKI, Japan). The virus induced CPE was scored on Day 3 post infection (p.i.) for HSV and influenza A and Day 5 p.i. for RSV. The reduction of virus multiplication was calculated as the percentage of virus control: virus control (%)=CPE exp/CPE virus control×100). The 50% effective concentration (EC50) was estimated from the plots graphically.
Plaque reduction assay
The antiviral activity of the aqueous extract against HSV was also determined by plaque reduction assay (PRA) and performed as described previously (Li et al., 2005) but with some modification. Briefly, confluent Vero cells in a 12-well plate (IWAKI, Japan) were infected with 80 plaque forming units per well (PFU per well) of HSV with or without (as control) the extract solutions. After adsorption at 37 °C for 1 h, residual inoculum was replaced with 1 ml of MM containing 0.6% (w/v) agarose and corresponding extract solution. These were incubated for 3 d to allow plaque formation. The EC50 was then estimated from the plots graphically.
Flow cytometric analysis
In this assay, Vero cells were infected by HSV in the presence of different concentrations of the carbohydrate fraction of algal extracts. All of the cells were then gathered, fixed and stained firstly with mouse anti-human HSV-1 (clone H62) or HSV-2 (clone HH-2) monoclonal antibody (Argène-Biosoft, Varihles, France), and then with FITC-conjugated goat anti-mouse IgG polyclonal antibodies (PharMingen, USA). The stained cells were then subjected to analysis with BECKMAN EPICS XL Flow Cytometry System (USA). Fluorescein-5-isothiocyanate (FITC)-fluorescence from the anti-HSV antibody was measured at photomultiplier 1 (PMT1, 525 nm) (Chi-Ming Chiu et al., 2004).
RESULTS
Cellular toxic effect of extracts
When different cells were exposed to the extracts at the final concentration ranging from 31.25 μg/ml to 1 000 μg/ml for 72 h separately, the hot water extract of S. hemiphyllum showed the highest toxicity on Vero cells. By observing the morphological changes of cells, extracts from the other five algae had moderate to low cytotoxicity. The MNCC of these extracts were all higher than 250 μg/ml. The extracts of D. dichotoma and P. australis were only slightly toxic to Vero cells with high CC50 values of 925 and 1 000 μg/ml, respectively (Table 1). The extracts of H. clathratus and L. variegata also showed low toxic effect on HEp-2 and MDCK cells, with the CC50 values being higher than 500 μg/ml for both these cell lines.
Table 1.
Cellular toxicity of aqueous extracts of Hong Kong seaweeds on Vero, HEp-2 and MDCK cells
| Seaweed species |
MNCC (μg/ml) |
CC50 (μg/ml) |
||||
| Vero cell | HEp-2 cell | MDCK cell | Vero cell | HEp-2 cell | MDCK cell | |
| A | 250 | ND | ND | 450 | ND | ND |
| B | 500 | ND | ND | 925 | ND | ND |
| C | 500 | 250 | 500 | 780 | 560 | 780 |
| D | 400 | 500 | 500 | 700 | 620 | >1 000 |
| E | 750 | ND | ND | 1 000 | ND | ND |
| F | 62.5 | ND | ND | 125 | ND | ND |
A: Colpomenia sinuosa; B: Dictyota dichotoma; C: Hydroclathrus clathratus; D: Lobophora variegata; E: Padina australis; F: Sargassum hemiphyllum. MNCC: maximum non-cytotoxic concentration on cells; CC 50: the concentration that reduced 50% of viable cells tested by MTT method; ND: not detected. All data were the means of two separate tests
Antiviral activity assay
The six crude extracts were studied for their inhibitory effect on HSV replication in Vero cells firstly by measuring the CPE reduction. The monolayers of Vero cells in 96-well plates were infected with HSV-1 and HSV-2 at the multiplicity of infection (MOI) of 1 000 TCID50/ml in the absence or presence of serially twofold diluted crude extracts. The CPE induced by the virus was scored on Day 3 p.i.
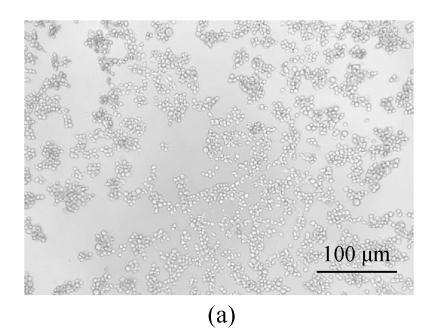
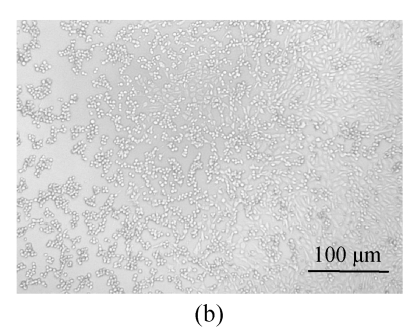
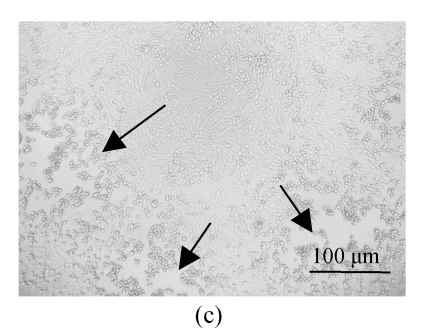
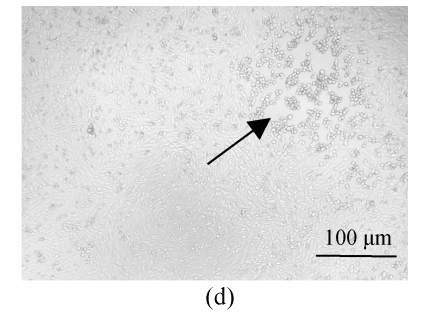
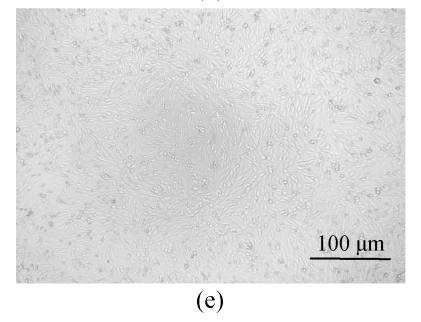
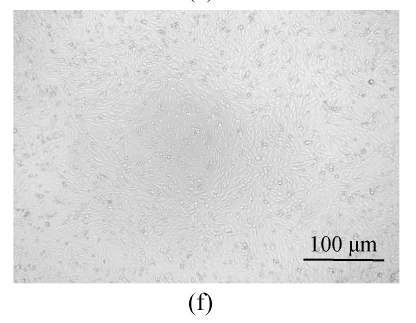
All of the seaweed extracts could, in different degrees, protect the Vero cells from the infection of HSV-1 and HSV-2. The extract of H. clathratus had the highest antiviral effect on HSV-1 and HSV-2. This is followed by the extract of L. variegata. The others were moderate anti-HSV agents. When observed under the inverted microscope, the CPE induced by HSV decreased after being treated by these extracts. Fig.1 shows the reduction of CPE induced by HSV-1 in Vero cells after being treated with various concentrations of H. clathratus extract for 3 d. The effect was concentration-dependent, with less and less CPE developed when compared with the viral control that showed 100% CPE (Fig.1a) as the extract concentration increased. At the dose of 25 μg/ml (Fig.1e), no CPE appeared and growth of the host cells was the same as that of the control cells (Fig.1f). Similar CPE reduction was also observed in Vero cells infected by HSV-2 and treated with H. clathratus extract (data not shown).
Fig. 1.
CPE reduction assay showing the inhibitory effect of H. clathratus extract on HSV-1
Vero cells were infected with HSV-1 15577 strain and treated with the test sample at the final concentrations of (a) 0 μg/ml (acted as viral control), (b) 3.1 μg/ml, (c) 6.25 μg/ml, (d) 12.5 μg/ml, and (e) 25 μg/ml. The cell control is exhibited in (f). The arrows point out distinct CPE induced by the virus
These results were confirmed and quantified by plaque reduction assay. Among the six seaweeds, the extract of H. clathratus had the highest anti-HSV activity with the lowest EC50 values of 6.25 μg/ml against the standard strains of HSV-1 and HSV-2 (Table 2). Based on its low cytotoxicity and high antiviral effect, the selective index (SI) of H. clathratus extract was the highest. This is followed by the extract of L. variegata that showed higher antiviral effect especially against HSV-2 (Table 2). For its high cellular toxicity, the extract of S. hemiphyllum gave a low SI value indicating that its antiviral activity was related to its cytotoxicity to the host cells.
Table 2.
Anti-HSV activities of aqueous extracts of seaweeds tested by plaque reduction assay (PRA)
| Extracts of Seaweeds |
EC50 (μg/ml) |
SI |
||
| HSV-1 | HSV-2 | HSV-1 | HSV-2 | |
| A | 22.1 | 12.5 | 20.4 | 36.0 |
| B | 24.3 | 25.0 | 38.1 | 24.3 |
| C | 6.25 | <6.25 | 124.8 | >124.8 |
| D | 18.5 | 9.0 | 37.8 | 77.8 |
| E | 58.9 | 40.0 | 17.0 | 25.0 |
| F | 19.1 | 12.5 | 6.5 | 10.0 |
A: Colpomenia sinuosa; B: Dictyota dichotoma; C: Hydroclathrus clathratus; D: Lobophora variegata; E: Padina australis; F: Sargassum hemiphyllum. SI: selective index, the value of CC 50/EC 50; HSV-1, HSV-2: antiviral effect on standard strain of HSV-1 and HSV-2. All data were the means of three repeated tests
The extracts of H. clathratus and L. variegata also showed some inhibitory effect against RSV with the EC50 of 25 and 100 μg/ml, respectively. But both of them had no anti-influenza A virus effect even at the concentration of 200 μg/ml (Table 3).
Table 3.
Antiviral activities of extracts from H. clathratus and L. variegata on RSV and influenza A virus
| Viruses |
EC50 (μg/ml) |
|||
| H. clathratus | L. variegata | DS | Ribavirin | |
| RSV* | 25 | 100 | 3.1 | 3.0 |
| Influenza A* | NA | NA | ND | 5.0 |
MOI=100 TCID50/ml;
NA: no activity at concentrations up to 200 μg/ml; ND: not detected
The extracts of H. clathratus and L. variegata showed excellent antiherpetic activities, including the inhibitory ability on acyclovir (ACV)-resistant HSV-1 (DM2.1 strain) and the clinical strains. Moreover, these two crude extracts exhibited a slightly higher effect on HSV-2 than on HSV-1 (Table 4).
Table 4.
Antiviral activities of extracts from H. clathratus and L. variegata on various strains of HSV as tested by PRA method on Vero cells
| Viruses |
EC50 (μg/ml) |
|||
| H. clathratus | L. variegata | DS | ACV | |
| A | 6.25 | 18.5 | 1.6 | 0.125 |
| B | 6.25 | 18.2 | ND | ND |
| C | 9.0 | 12.5 | ND | 0.125 |
| D | <6.25 | 9.0 | 0.8 | ND |
| E | <6.25 | 6.25 | ND | ND |
A: HSV-1 standard strain; B: HSV-1 DM2.1 strain; C: HSV-1 clinical strain; D: HSV-2 standard strain; E: HSV-2 clinical strain. The data were the means of two repeated tests; ND: not detected
Hydroclathrus clathratus was extracted by hot water, diluted acid and alkali solution, and four components were obtained. Two fractions precipitated from aqueous extract by 30% and 70% (v/v) ethanol were named as HI-2 and HI-3, respectively, and another two fractions HII-A and HIII-A were obtained from acid and alkali solution extracts, respectively.
The results listed in Table 5 show that most of the carbohydrates in H. clathratus could be extracted by hot water. This part also showed the highest antiviral effect against HSV-1 and HSV-2 when compared with the acid and alkali extracts. HI-3 precipitated by 70% (v/v) of ethanol from water crude extract had excellent inhibitory effects against both HSV-1 and HSV-2 with very low EC50 values at 1.6 and 0.8 μg/ml, respectively (Table 5). This is comparable to that of the positive control compound DS (Table 4).
Table 5.
Inhibitory effects of crude water extract and other four fractions isolated from H. clathratus on plaque formation of HSV-1 and HSV-2 standard strain in Vero cells
| Fractions | Yield rate (%)* |
EC50 (μg/ml) |
|
| HSV-1 15577 strain | HSV-2 8702 strain | ||
| Water crude extract | 2.030 | 6.0 | 3.1 |
| HI-2 | 0.360 | 6.5 | 3.1 |
| HI-3 | 0.780 | 1.6 | 0.8 |
| HII-A | 0.034 | 6.3 | 1.6 |
| HIII-A | 0.187 | 6.3 | 3.1 |
Yield rate was calculated based on wet weight of the seaweed samples
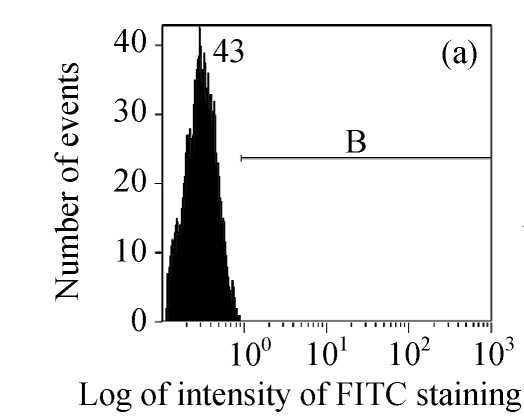
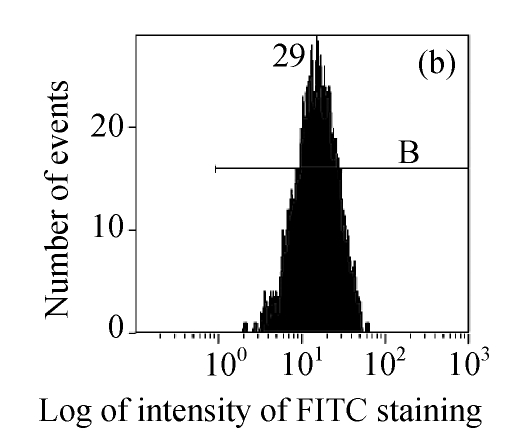
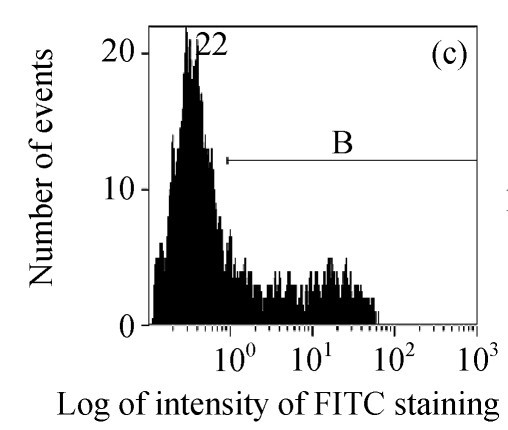
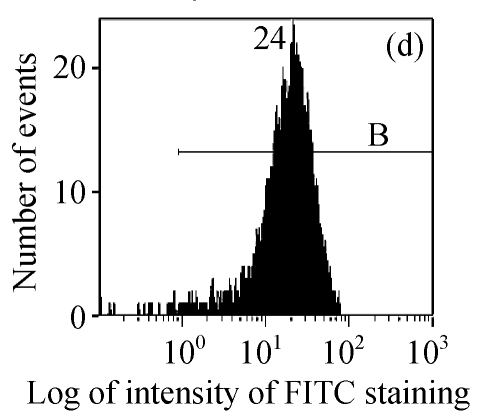
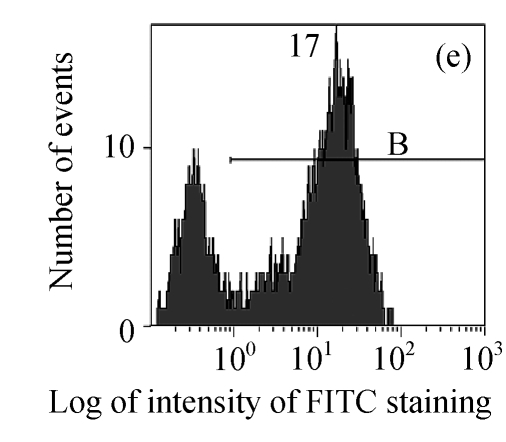
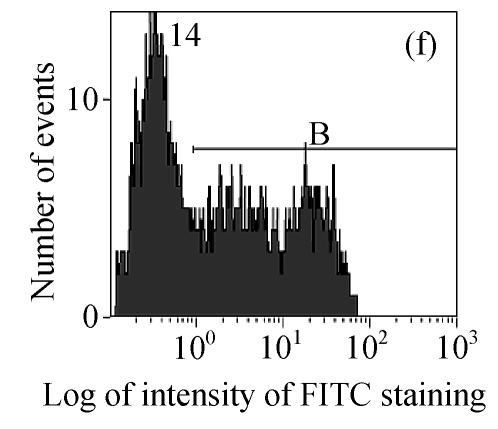
The efficacy value for HI-3 against HSV was also obtained from flow cytometric assay, and the FITC-fluorescence level from the anti-HSV antibody in the cells was measured. The EC50 values of HI-3 determined by flow cytometry were statistically similar to those obtained by plaque reduction assay. Fig.2 illustrates the typical histogram of fluorescence level in Vero cells exposed to HSV-1 15577 strain under the treatment with different concentrations of HI-3. Compared with the results of cell control (Fig.2a), virus control (Fig.2b) and the dextran sulfate positive control (Fig.2c), the replication of HSV-1 15577 strain was repressed by a dose-dependent response to HI-3 (Figs.2d~2f). HI-3 could therefore protect the Vero cells from the infection of HSV. The higher the concentration of this extract presented, the less the amount of viral antigen could be detected in the infected host cells. HI-3 also showed an obvious dose-related antiviral effect against HSV-2 8702 strain when similarly tested by flow cytometry. The resulting histograms depicting FITC-fluorescence level from the anti-HSV-2 antibody are similar to those shown in Fig.2 and are therefore no longer shown here.
Fig. 2.
Antiviral effect of HI-3 against standard strain of HSV-1 as analyzed by flow cytometric system
These histograms depict FITC-fluorescence level from the anti-HSV-1 antibody in (a) cell control, (b) HSV-1 control, and HSV-1-infected Vero cells that had been treated with (c) 3.13 μg/ml of dextran sulfate (positive control), or (d) 1.56 μg/ml HI-3, (e) 3.13 μg/ml HI-3, (f) 6.25 μg/ml HI-3. In each histogram, B bar indicates the proportion of cells that were positive for the HSV-1 antigen
DISCUSSION
Until now, chemically synthesized or modified compounds have been the major source of selective antiviral agents, particularly in the case of antiherpetic compounds (Alché et al., 2002). For combating drug resistant viral strain, it has been suggested that the new antiherpes drugs should be non-nucleosides. Thus, many efforts have been carried out to screen for antiviral agents from natural sources. Marine algae have shown their potential as important sources of antiviral as well as other bioactive compounds (Ahn et al., 2002).
In this study, we examined hot water extracts of six species of brown seaweeds and found that they showed antiviral activities against herpes simplex virus and had low cytotoxicity to Vero cells, except S. hemiphyllum extract. These extracts could inhibit the replication of various strains of HSV, including the ACV-resistant strain. Earlier works also showed that the aqueous extracts from five out of eight species of Hong Kong seaweeds exhibited some potential antiviral activities and very low cytotoxicity (Zhu, 2002). These studies indicate that the antiviral activities of these seaweed extracts were not due to their cytotoxic effect on host cells. Therefore, Hong Kong seaweeds could be a promising source of natural products with antiherpetic effect at non-toxic concentrations, just like seaweeds from other geographical regions.
It has been reported that seaweed extracts can be active against various enveloped viruses, including DNA and RNA viruses such as human immunodeficiency virus (HIV), HSV, cytomegalovirus (CMV), RSV and influenza virus (Baba et al., 1988). In the present investigation, the best inhibitory effect on HSV was shown by the hot water extract of H. clathratus, followed by that from L. variegata. They also showed some anti-RSV activities in HEp-2 cells but could not inhibit influenza A virus in MDCK cells even at a high concentration (200 μg/ml). HSV is an enveloped DNA virus while RSV and influenza A are both enveloped RNA viruses. Influenza A contains segmented genome. Therefore, it seems that the extracts from these brown seaweeds could exhibit higher antiviral effect on enveloped DNA virus than on RNA virus, and had no effect on RNA virus with segmented genome, e.g., the influenza virus. However, Damonte et al.(1994) reported that the water extract of a red seaweed showed high inhibitory effect on RSV in HeLa cells and influenza A virus in MDCK cells with very low EC50 values. These findings suggest that there are possible differences in the virus-inhibitory effects of the seaweed extract. These differences may not only depend on the choice of cell lines used in the assay, but also on the seaweed species collected from different habitats, geographical regions or even at different collecting time (Zhu, 2002).
All the six crude extracts in the current screening showed positive results in phenol-sulfuric acid reaction (data not shown), suggesting that the main effective components in these extracts were polysaccharides. As can be found in the literature, a number of polyanionic substances or water-soluble sulfated polysaccharides are potential antiviral agents (Béress et al., 1993; Ponce et al., 2003). It is commonly suggested that these negatively charged molecules disturb the proliferation cycle of enveloped viruses at the early stage, therefore inhibiting the viral infection (Witvrouw and de Clercq, 1997). However, Nakashima et al.(1987) found that a sulfated seaweed polysaccharide selectively inhibited reverse transcriptase (RT) enzyme of human immunodeficiency virus (HIV) and its replication in vitro. Other studies showed that sulfated polysaccharides could prevent viral protein synthesis (González et al., 1987) or were capable of blocking various steps during the life cycle of HSV (Huleihel et al., 2001). The pleiotropic modes of antiviral actions of sulfated polysaccharides make it less likely for the virus to develop resistant mutants.
The carbohydrates contained in seaweed could be extracted by water, diluted acid and alkali (Witvrouw and de Clercq, 1997). In the present experiment, these three parts of carbohydrates from H. clathratus were tested. They all showed antiviral activities against HSV-1 and HSV-2 and the largest portion, the hot water extract, has the most potential for developing into antiviral agent. HI-3, which was fractionated from the hot water extract, exhibited an excellent effect in antiviral test using both the plaque reduction and flow cytometric assays. Further fractionation of HI-3 yielded two different sulfated polysaccharides. These polysaccharides showed comparable antiviral activities. Detailed performances of these polysaccharides and their characterization were reported in Wang et al.(2007b). Some seaweed polysaccharides, e.g., dextran sulfate and heparin, have been shown to inhibit the replication of various enveloped viruses, including HSV and HIV (Witvrouw and de Clercq, 1997). The modes of action of most of these polysaccharides have often been attributed to a blockade of the early stages of the virus replication cycle (Bourne et al., 1999). The polysaccharides isolated from H. clathratus could have the same anti-HSV action as that shown by dextran sulfate, but more details of their antiviral mechanism are still being evaluated.
Footnotes
Project (No. CUHK4367/01M) supported by the Research Grants Council of the Hong Kong SAR, China
References
- 1.Ahn MJ, Yoon KD, Kim CY, Min SY, Kim Y, Kim HJ, Kim JH, Shin CG, Lee CK, Kim TG, et al. Inhibition of HIV-1 reverse transcriptase and HIV-1 integrase and antiviral activity of Korean seaweed extracts. J Appl Phycol. 2002;14(5):325–329. doi: 10.1023/A:1022192329471. [DOI] [Google Scholar]
- 2.Alché LE, Barquero AA, Sanjuan NA, Coto CE. An antiviral principle present in a purified fraction from Melia Azedarach L. leaf aqueous extract restrains herpes simplex virus type-1 propagation. Phytother Res. 2002;16(4):348–352. doi: 10.1002/ptr.895. [DOI] [PubMed] [Google Scholar]
- 3.Ammendolia MG, Marchetti M, Superti F. Bovine lactoferrin prevents the entry and intercellular spread of herpes simplex virus type 1 in Green Monkey Kidney cells. Antiviral Res. 2007;76(3):252–262. doi: 10.1016/j.antiviral.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Ang PO. Studies of Marine Algae in Hong Kong. In: Critichley AT, Ohno M, Largo D, editors. World Seaweed Resources. Wokingham, Berkshire, UK: ETI Information Services Ltd; 2005. Part 3.04. [Google Scholar]
- 5.Baba M, Snoeck R, Pauwels R, de Clercq E. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32(11):1742–1745. doi: 10.1128/aac.32.11.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker Y. Past, present and future of RSV and PIV vaccines and anti-RSV antibodies for the protection of humans against RSV. Expert Opin Ther Pat. 2007;17(8):941–953. doi: 10.1517/13543776.17.8.941. [DOI] [Google Scholar]
- 7.Béress A, Wassermann O, Bruhn T, Béress L. A new procedure for the isolation of anti-HIV compounds (polysaccharides and polyphenols) from the marine alga Fucus vesiculosus . J Nat Prod. 1993;56(4):478–488. doi: 10.1021/np50094a005. [DOI] [PubMed] [Google Scholar]
- 8.Bourne KZ, Bourne N, Reising SF, Stanberry LR. Plant products as topical microbicide candidates: assessment of in vitro and in vivo activity against herpes simplex virus type 2. Antiviral Res. 1999;42(3):219–226. doi: 10.1016/S0166-3542(99)00020-0. [DOI] [PubMed] [Google Scholar]
- 9.Burleson FG, Chambers TM, Wiedbrauk DL. Virology: A Laboratory Manual. California, San Diego: Academic Press; 1992. pp. 53–61. [Google Scholar]
- 10.Chi-Ming Chiu L, Zhu W, Eng-Choon Ooi V. A polysaccharide fraction from medicinal herb Prunella vulgaris downregulates the expression of herpes simplex virus antigen in Vero cells. J Ethnopharmacol. 2004;93(1):63–68. doi: 10.1016/j.jep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Chutkowski C, Olson B, McDonough A, Mahoney J, McSharry JJ. Use of a single monoclonal antibody to determine the susceptibilities of herpes simplex virus type 1 and type 2 clinical isolates to acyclovir. Clin Diagn Lab Immunol. 2002;9(6):1379–1381. doi: 10.1128/CDLI.9.6.1379-1381.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Damonte E, Neyts J, Pujol CA, Snoeck R, Andrei G, Ikeda S, Witvrouw M, Reymen D, Haines H, Matulewicz MC, et al. Antiviral activity of a sulphated polysaccharide from the red seaweed Nothogenia fastigiata . Biochem Pharmacol. 1994;47(12):2187–2192. doi: 10.1016/0006-2952(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 13.de Clercq E. Antiviral and antimetabolic activities of neplanocins. Antimicrob Agents Chemother. 1985;28(1):84–89. doi: 10.1128/aac.28.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Clercq E. Perspectives for the chemotherapy of respiratory syncytial virus (RSV) infections. Int J Antimicrob Agents. 1996;7(3):193–202. doi: 10.1016/S0924-8579(96)00319-6. [DOI] [PubMed] [Google Scholar]
- 15.González ME, Alarcón B, Carrasco L. Polysaccharides as antiviral agents: antiviral activity of carrageenan. Antimicrob Agents Chemother. 1987;31(9):1388–1393. doi: 10.1128/aac.31.9.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huleihel M, Ishanu V, Tal J, Arad S. Antiviral effect of red microalgal polysaccharides on herpes simplex and Varicella zoster viruses. J Appl Phycol. 2001;13(2):127–134. doi: 10.1023/A:1011178225912. [DOI] [Google Scholar]
- 17.Kneyber MCJ, Moll HA, de Groot R. Treatment and prevention of respiratory syncytial virus infection. Eur J Pediatr. 2000;159(6):399–411. doi: 10.1007/s004310051296. [DOI] [PubMed] [Google Scholar]
- 18.Li YL, But PPH, Ooi VEC. Antiviral activity and mode of action of caffeoylquinic acids from Schefflera heptaphylla (L.) Frodin. Antiviral Res. 2005;68(1):1–9. doi: 10.1016/j.antiviral.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Mayer AMS, Lehmann VKB. Marine pharmacology in 1998: marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities; with actions on the cardiovascular, endocrine, immune, and nervous systems, and other miscellaneous mechanism of action. The Pharmacologist. 2000;42:62–69. [Google Scholar]
- 20.Nakashima H, Kido Y, Kobayashi N, Motoki Y, Neushul M, Yamamoto N. Prification and characterization of an avian myeloblastosis and human immunodeficiency virus reverse transcriptase inhibitor, sulfated polysaccharides extracted from sea algae. Antimicrob Agents Chemother. 1987;31(10):1524–1528. doi: 10.1128/aac.31.10.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ponce NMA, Pujol CA, Damonte EB, Flores ML, Stortz CA. Fucoidans from the brown seaweed Adenocystis utricularis: extraction methods, antiviral activity and structural studies. Carbohydr Res. 2003;338(2):153–165. doi: 10.1016/S0008-6215(02)00403-2. [DOI] [PubMed] [Google Scholar]
- 22.Scott PD, Ochola R, Sande D, Ngama M, Okiro EA, Medley GF, Nokes DJ, Cane PA. Comparison of strain-specific antibody responese during primary and secondary infections with respiratory syncytial virus. J Med Virol. 2007;79(12):1943–1950. doi: 10.1002/jmv.20999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serkedjieva J, Ivancheva S. Antiherpes virus activity of extracts from the medicinal plant Geranium sanguineum L. J Ethnopharmacol. 1998;64(1):59–68. doi: 10.1016/S0378-8741(98)00095-6. [DOI] [PubMed] [Google Scholar]
- 24.Wang H, Li YL, Shen WZ, Rui W, Ma XJ, Cen YZ. Antiviral activity of a sulfoquinovosyldiacylglycerol (SQDG) compound isolated from the green alga Caulerpa racemosa . Botanica Marina. 2007;50(3):185–190. doi: 10.1515/BOT.2007.022. [DOI] [Google Scholar]
- 25.Wang H, Ooi ECV, Ang POJr. Antiviral polysaccharides isolated from Hong Kong brown seaweed Hydroclathrus clathratus . Sci China C Life Sci. 2007;50(5):611–618. doi: 10.1007/s11427-007-0086-1. [DOI] [PubMed] [Google Scholar]
- 26.Witvrouw M, de Clercq E. Sulfated polysaccharides extracted from sea algae as potential antiviral drugs. Gen Pharmacol. 1997;29(4):497–511. doi: 10.1016/S0306-3623(96)00563-0. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W. Antiviral Activities of Selected Hong Kong Marine Algae against Herpes Simplex Viruses and Other Viruses and Their Possible Antiviral Mechanisms. Hong Kong, China: the Chinese University of Hong Kong; 2002. pp. 186–188. (PhD Thesis) [Google Scholar]
- 28.Zhu W, Ooi VEC, Chan PKS, Ang POJr. Isolation and characterization of a sulfated polysaccharide from the brown alga Sargassum patens and determination of its anti-herpes activity. Biochem Cell Biol. 2003;81(1):25–33. doi: 10.1139/o02-169. [DOI] [PubMed] [Google Scholar]