Abstract
Transgenic female mice expressing the TNFα gene under the cardiac myosin promoter (TNF1.6) develop substantially increased myocarditis and increased numbers of CD4+Th1 (interferon gamma+) cells when infected with coxsackievirus B3 (CVB3) during the diestrus and proestrus phases of the estrus cycle compared to females infected during the estrus and metestrus phases. Cardiac virus titers were increased in females infected in estrus compared to females infected during the other phases. T regulatory cells (CD4+CD25+FoxP3+) were increased in both peripheral blood and inflammatory cells in the heart in females infected during estrus. Exogenous administration of 200 ng/mouse 17-β-estradiol to females protected against CVB3 induced myocarditis and increased CD4+CD25+FoxP3+ cells. These results demonstrate that hormonal fluctuations occurring in normally cycling females can determine T regulatory cell response and control virus-induced pathogenesis.
Keywords: Coxsackievirus, Myocarditis
INTRODUCTION
Coxsackievirus B3 (CVB3) is a member of the picornavirus family of small, non-enveloped, positive sense, RNA viruses (Rueckert, 1996), and are major etiological agents in clinical viral myocarditis (Bowles et al., 2003; Bowles and Towbin, 2000). A murine model of CVB3 induced myocarditis has been established which mimicks many of the characteristics of the clinical disease (Gauntt and Huber, 2003; Gauntt et al., 2000; Huber, Gauntt, and Sakkinen, 1999; Woodruff, 1980). One aspect of clinical myocarditis is than men are more likely to develop the disease than women, except during the third trimester of pregnancy (Woodruff, 1980). Similarly, in the murine model, virgin female mice are generally protected from CVB3 induced myocarditis compared to males and females in the third trimester of pregnancy (Huber, Job, and Auld, 1982; Huber, Kupperman, and Newell, 1999; Lyden and Huber, 1984). Sex-associated hormones are predominantly responsible for the gender difference in myocarditis susceptibility with androgens (testosterone and progesterone) promoting disease and estrogen inhibiting it (Huber, Job, and Auld, 1982; Lyden and Huber, 1984). Recently, this laboratory demonstrated that virgin female susceptibility depends upon the phase of the estrus cycle when infection occurs. Females infected in diestrus when progesterone levels are naturally elevated and estradiol levels are reduced results in increased myocarditis compared to females infected during estrus when estradiol levels are elevated (Schwartz, Sartini, and Huber, 2004). The effect of infecting mice in different phases of the estrus cycle was consistent but even females infected during diestrus never achieved the myocarditis severity observed in males or pregnant females. The increased susceptibility in females infected during diestrus correlated to a preferential CD4+Th1 (IFNγ+) cell response while females infected during estrus showed minimal CD4+ Th1 cells but increased CD4+Th2 (IL-4+) cells. Sex-associated hormones are well-known to impact the innate and adaptive immune response. Hormonal environment can alter dendritic cell maturation and antigen presentation, cytokine/chemokine expression, and Toll-like receptors (TLR) expression, all of which can have substantial affects on developing immunity (Aflatoonian et al., 2007; Carreras et al., 2008; Ito et al., 2001; Ito et al., 2002; Kovats and Carreras, 2008). Thus, it is not surprising that the fluctuating hormonal environment as found during the menstrual cycle and pregnancy influences immunity to microbial infections, both in the genital tract and systemically (Brabin, 2002). Several studies have shown that hormones modulate T regulatory cell responses (Polanczyk et al., 2005; Tai et al., 2008) by promoting conversion of CD4+CD25- cells to the regulatory CD4+CD25+ phenotype. The mechanism appears to be through estrogen receptors expression on the regulatory cell precursor and conversion is suppressed by the estrogen receptor. As T regulatory cells inhibit CD4+Th1 cell responses (Nie et al., 2007), which are essential to CVB3 induced myocarditis susceptibility (Huber, Shi, and Budd, 2002; Huber, Sartini, and Exley, 2002), we hypothesize that infection of females during the ovarian cycle phases known to have high estrogen concentration would preferentially activate T regulatory cell responses and suppress Th1 cell dependent diseases.
RESULTS
CVB3 infection increases myocarditis in females infected in diestrus and proestrus phases of the ovarian cycle
The first experiment determined whether estrus cycle affected heart weight and heart weight:body weight ratios in uninfected female FVB TNF1.6 transgenic mice. Female TNF1.6 mice (6–8 weeks of age) were subjected to vaginal smears and killed immediately to ensure that the heart and body weights reflected the estrus cycle at the time of euthanasia. Animals were weighed. Hearts were removed and weighed (Figure 1). There was no significant difference in heart weight or in heart:body weight ratios in uninfected mice in any of the phases of the estrus cycle. This demonstrates that the estrus cycle does not directly alter cardiomyopathy induced by TNFα expression under the cardiac myosin promoter. Next, females were infected immediately after the vaginal smear to ensure that infection occurred specifically in the estrus phase indicated. Seven days after infection, mice were killed and hearts were removed and weighed (Figure 2). Unlike uninfected TNF1.6 mice, infection during diestrus and proestrus phases results in significantly higher heart weights than infection during estrus and metestrus. This corresponds to substantial increases in cardiac inflammation in mice infected in diestrus and proestrus (Figure 3 and Figure 4). Cardiac virus titers do not correlate to cardiac inflammation since maximum virus occurred in mice infected during estrus and was significantly reduced when mice were infected in metestrus.
Figure 1.
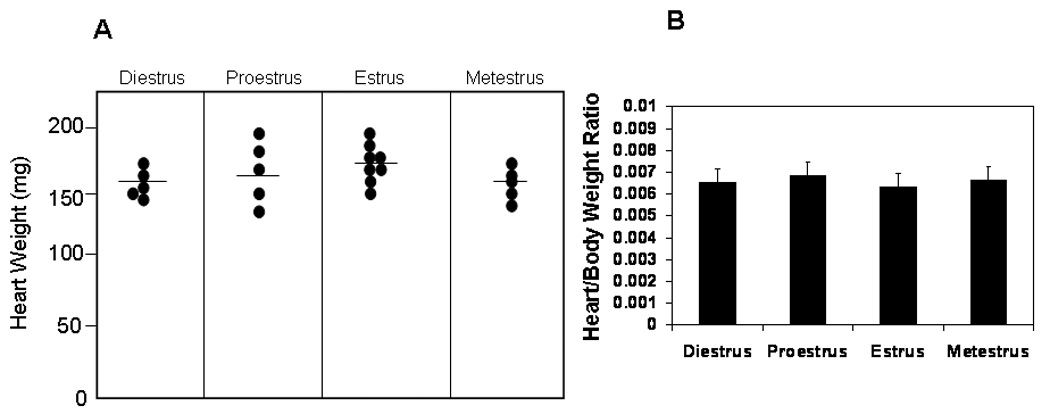
Phase of the estrus cycle has no effect on heart weight or heart:body weight ratios in uninfected TNF1.6 female mice. Vaginal smears were done and the mice killed immediately. Animals were weighed. Hearts were perfused with PBS, removed, dried and weighed. (A) Each point represents the weight of the heart from an individual animal. Bar indicates mean weight for the group. (B) Mean ± SEM heart:body weight ratio of mice given in (A).
Figure 2.
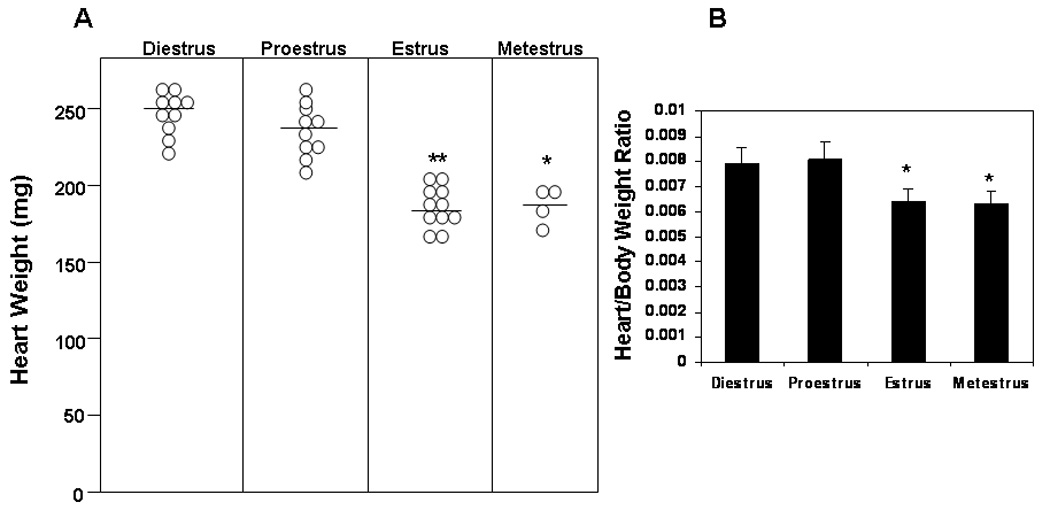
Female TNF1.6 mice infected with CVB3 in diestrus and proestrus phases of the estrus cycle develop more severe cardiomyopathy. Vaginal smears were done and mice infected with 105 PFU CVB3 i.p. immediately. Mice were killed 7 days after infection and weighed. Hearts were perfused with PBS, removed, dried and weighed. (A) Each point represents an individual animal. Bar indicates mean weight for the group. (B) Mean ± SEM heart:body weight ratio of mice given in (A). ** Different from mice infected in diestrus at p≤ 0.01.
Figure 3.
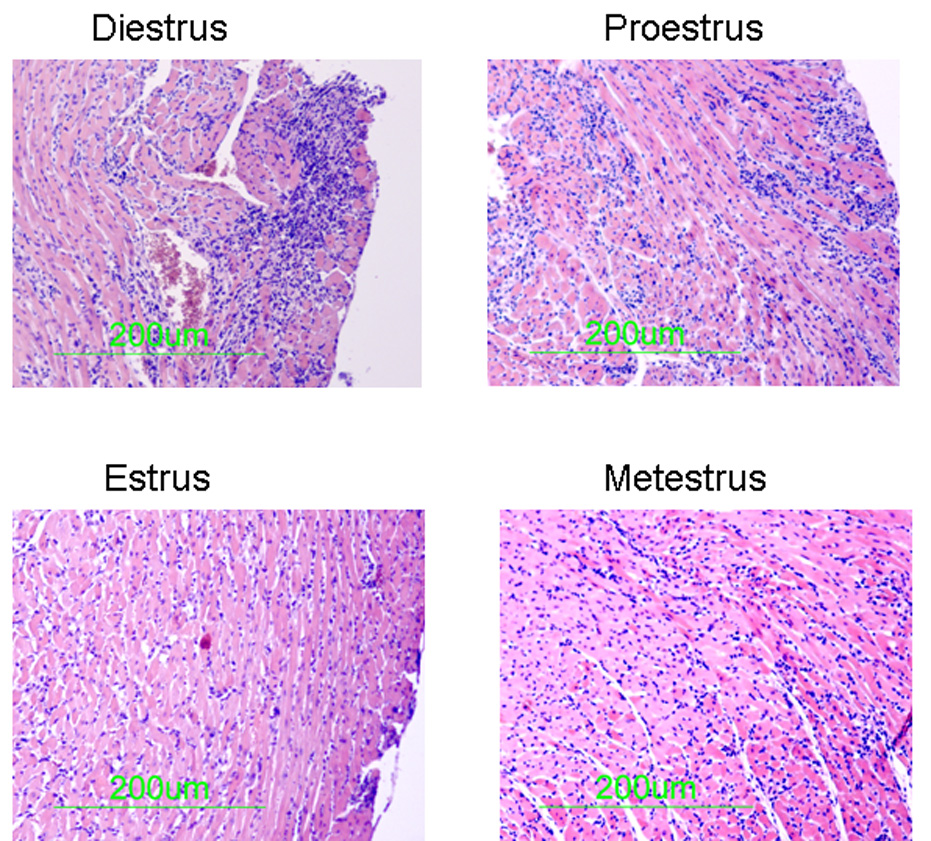
Histology of TNF1.6 mice infected in different phases of the estrus cycle. Vaginal smears were performed and mice were immediately infected i.p. with 105 PFU CVB3. 7 days later, animals were killed and hearts were removed, formain fixed, paraffin embedded, sectioned and stained with hematoxylin and eosin. Photomicrographs are representative of mice in the group.
Figure 4.
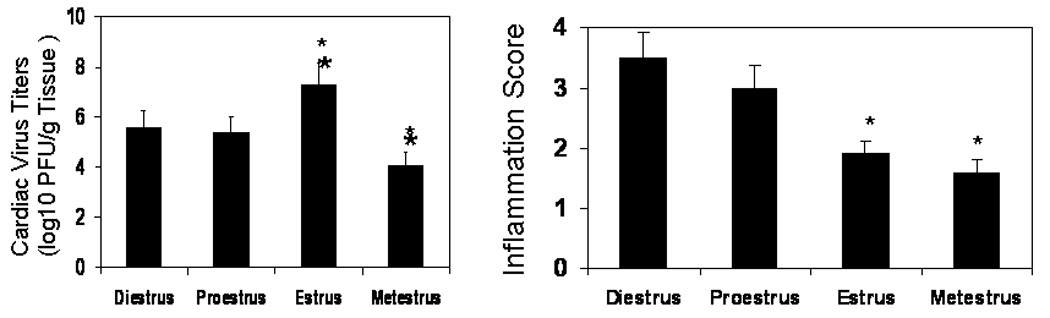
Evaluation of cardiac virus titers and myocarditis in female mice infected with CVB3 during different phases of the estrus cycle. Vaginal smears were done on TNF1.6 female mice and the animals were immediately infected with 105 PFU CVB3 then killed 7 days later. Hearts were evaluated for virus titer using the plaque forming assay for myocardial inflammation using a 0 to 4 grading scale. Results represent mean ± SEM of 4 to 11 mice/group (heart weights shown in Figure 2). * Significantly different than mice in diestrus at p< 0.05.
Myocarditis correlates to activated CD4+ and Vγ4+ cells and is inversely correlated to FoxP3+ T regulatory cells
Previous studies have shown that activated Vγ4+ cells and CD4+Th1 (IFNγ+) cells are essential to CVB3 induced myocarditis (Huber, Born, and O'Brien, 2005; Huber, Sartini, and Exley, 2002). Therefore, these cell populations were evaluated in females infected in different phases of the estrus cycle. Peripheral blood mononuclear cells (PBMC) were isolated from individual mice and labeled with antibodies to CD4, Vγ4, and either IFNγ or the early activation marker, CD69, as indications of T cell activation (Figure 5). Cells were also labeled with antibodies to CD4, CD25 and FoxP3 to identify T regulatory cells. Animals infected during diestrus and proestrus had significantly increased numbers of both activated CD4+ (CD4+CD69+ and CD4+IFNγ+) and Vγ4+ (Vγ4+IFNγ+) cells while these animals had reduced numbers of T regulatory cells compared to mice infected in estrus. T cells in peripheral blood potentially may not reflect inflammatory cell composition in the inflammatory site, in this case, in the heart. Therefore, hearts were perfused with PBS to remove peripheral blood, minced and enzymatically digested to release inflammatory cells. The cells were labeled with antibodies to CD4, Vγ4, IFNγ, IL-4 and CD4/CD25/FoxP3 (Figure 6). As in peripheral blood, increased numbers of CD4+IFNγ+ cells were observed in the hearts of mice infected in diestrus and proestrus while increased numbers of CD4+IL-4+ and CD4+CD25+FoxP3+ cells were found in hearts of mice infected in estrus. Data from both heart and peripheral blood indicates that activation of T regulatory cells depends upon the phase of the estrus cycle when females are virally infected.
Figure 5.
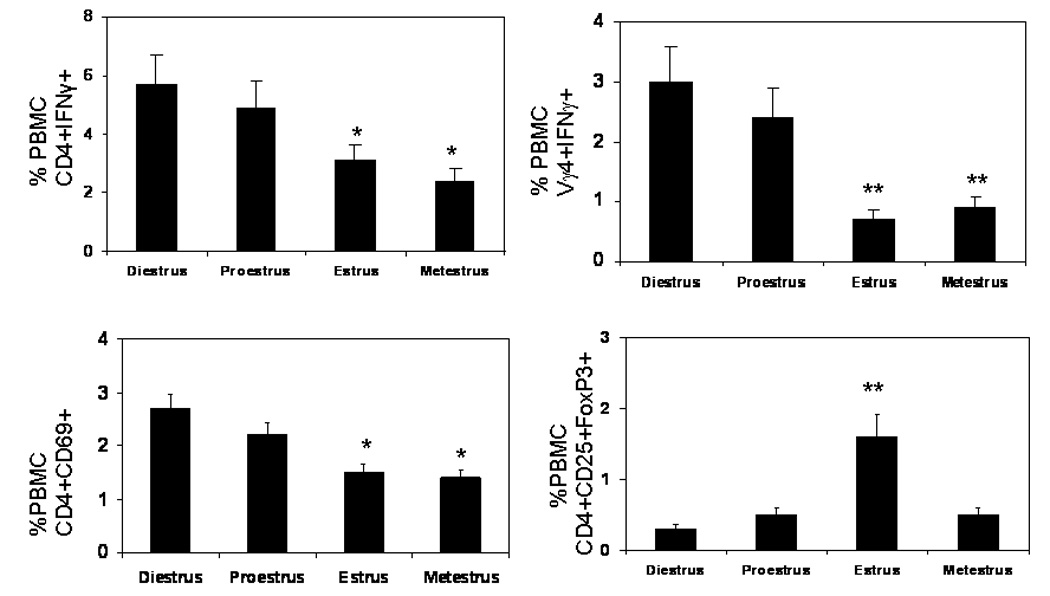
Evaluation of immune response of female mice infected with CVB3 during different phases of the estrus cycle. TNF1.6 mice were infected in different phases of the estrus cycle and killed 7 days later. Peripheral blood mononuclear cells (PBMC) were isolated from individual mice and evaluated by flow cytometry. Results represent mean ± SEM of 4 to 11 mice/group. * Significantly different from mice infected in diestrus at p< 0.05. ** Significantly different than mice infected in diestrus at p≤ 0.01.
Figure 6.
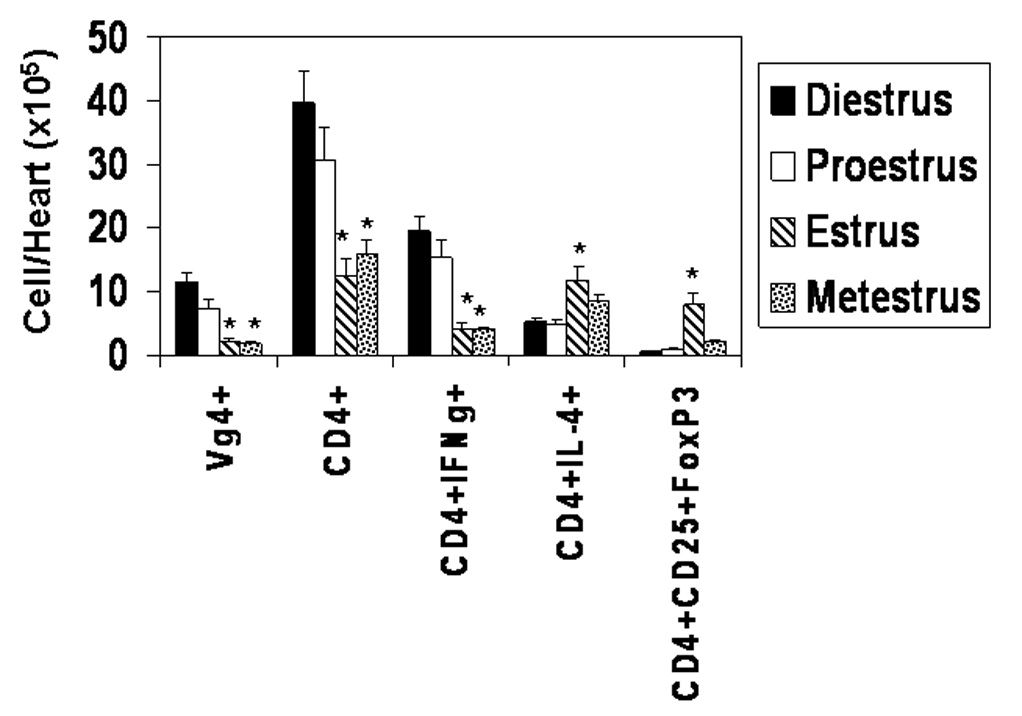
Characterization of the inflammatory cells infiltrating the hearts of females infected during different phases of the estrus cycle. Vaginal smears were done on female TNF1.6 mice and the animals were immediately infected with 105 PFU CVB3. The animals were killed 7 days later and the hearts were perfused with PBS, removed, dried, minced and digested with collagenase and pancreatin to retrieve inflammatory cells in the heart. The cells were counted by trypan blue exclusion and labeled with the indicated antibodies for flow cytometry analysis as described in the Materials and Methods. Number of cells represents the % of cells labeled for particular markers multiplied by the number of cells recovered from the heart. Results represent the mean ± SEM of 4 to 7 mice/group. * Significantly different from mice in diestrus at p< 0.05.
Role of estradiol in T regulatory cell activation
Previous studies indicate that relatively high levels of estrogen as seen during pregnancy can activate T regulatory cells (Polanczyk et al., 2005). Estradiol levels are lower during diestrus compared to estrus (Schwartz, Sartini, and Huber, 2004) and this might partially explain the increased T regulatory cell response observed when mice are infected in estrus. To demonstrate that estradiol can alter T regulatory cell response during CVB3 infection, mice were infected and subsequented injected i.p. with either PBS alone or PBS containing 200 ng 17-β-estradiol on days +1 and +3 relative to infection. Mice were killed 7 days after infection and evaluated for myocardial inflammation, cardiac virus titers and T regulatory cells in PBMC (Figure 7). Virus titers significantly increased in estradiol treated females infected in diestrus and metestrus. There was a slight, but not significant, increase in virus titers in estradiol treated females infected in proestrus. Myocarditis was significantly reduced in estradiol treated females in diestrus and proestrus. Estradiol treatment during early infection significantly increased T regulatory cell response in mice infected during diestrus, proestrus, and metestrus while having no effect in mice infected in estrus.
Figure 7.
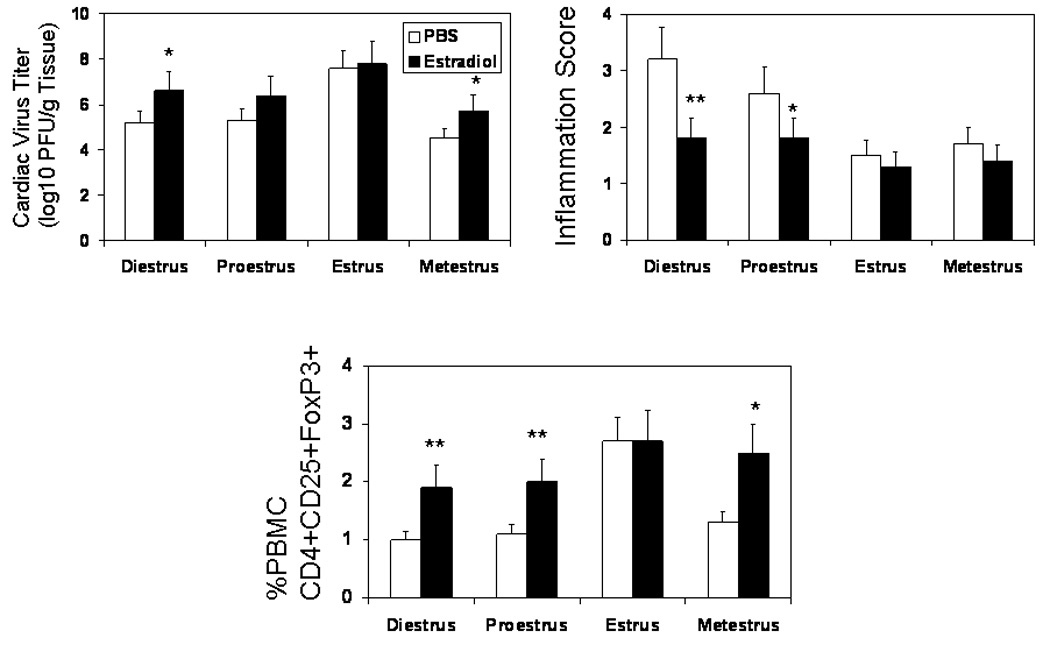
Estradiol treatment reduces myocarditis and increases FoxP3+ T regulatory cell response in female mice. Vaginal smears were done on TNF1.6 mice and the animals were immediately infected with 105 PFU CVB3. On days +1 and +3 relative to infection, mice were injected i.p. with 200 ng estradiol in PBS or PBS alone. Animals were killed 7 days after infection. Hearts were evaluated for virus titer and cardiac inflammation. PBMC were isolated from individual animals and evaluated for FoxP3+ T cells by flow cytometry. Results represent mean ± SEM of 4 to 6 mice/group. * Significantly different from mice in same estrus phase and treated with PBS at p< 0.05. ** Significantly different from mice in same estrus phase and treated with PBS at p≤ 0.01.
DISCUSSION
This study demonstrates that the phase of the ovarian cycle when females are infected can have a dramatic impact on anti-virus immunity and disease susceptibility due to preferential activation of T regulatory cells. Previous studies have investigated hormonal effects on T regulatory cell activation in pregnancy (Polanczyk et al., 2005), however, during pregnancy hormone levels are substantially elevated compared to levels normally observed in cycling females. The significance of the current work is that more modest hormonal fluctuations still have major impacts on immune regulation. Serum estradiol levels in diestrus are approximately 3 pg/ml compared to greater than 5 pg/ml in estrus. Levels in proestrus and metestrus average approximately 6 and 3 pg/ml, respectively (Schwartz, Sartini, and Huber, 2004). For three of the phases (diestrus, estrus and metestrus) T regulatory response correlates to relative estradiol levels in that phase being high estradiol level/high T regulatory cell in estrus but low estradiol/low T regulatory cell in diestrus and metestrus. An unexpected observation in this study was the low T regulatory cell response in proestrus when estradiol levels are elevated. Hormone levels can fluctuate rapidly in mice and females can move through the ovarian cycle within 1–2 to 10 days. However, mice underwent vaginal smears and infection almost simultaneously so that the smears should give an accurate reflection of the phase when infection occurred. One possibility is that while hormone levels change from phase to phase, the expression of the relevant factors controlling immunity, such as estrogen receptor or cytokine/chemokine expression, take more time. Animals in proestrus may not have up-regulated these factors yet despite increasing estradiol levels in the serum. Certainly, exogenous treatment of females in diestrus, proestrus and metestrus with estradiol substantially increased T regulatory cell activation during infection and inhibited myocarditis induction in diestrus and proestrus, confirming previous studies showing the ability of estrogen to promote immunoregulatory cells. In this model, as expected, there was an inverse correlation between the Th1 cell response induced by infection and the T regulatory cell numbers. The presence of T regulatory cells in mice infected during estrus would explain the lack of Th1 cell response and the preferential Th2 activation. Although activation of T regulatory cells is certainly one important factor determining myocarditis susceptibility, this cannot be the only one. Females infected in metestrus fail to develop myocarditis, but also have few detectable T regulatory (CD4+CD25+FoxP3+) cells. This suggests that other aspects of the metestrus environment control susceptibility. Previous studies have shown that exogenous administration of progesterone to virgin females aggravates CVB3 induced myocarditis to levels usually observed in infected males (Lyden and Huber, 1984). Progesterone levels during metestrus are low (<100 pg/ml plasma) compared to elevated levels (~200 pg/ml) during diestrus, proestrus and estrus (Schwartz, Sartini, and Huber, 2004). Therefore, this is the only phase in the ovarian cycle where the hormone associated with disease susceptibility is low. Low progesterone must prevent activation of CD4+ Th1 cells without involving T regulatory cells.
Disease susceptibility to a number of infectious agents is affected by the estrus cycle or sex-associated hormones. In dogs, the onset of pyometra, an infection of the uterus, occurs during diestrus (Sugiura et al., 2004). Female mice infected during estrus cycle phases with high progesterone are better able to clear Trypanosoma cruzi, the causative agent in Chagas’ Disease (Schuster and Schaub, 2001), and are also less susceptible to pseudorabies infection of the central nervous system than females infected when progesterone levels are low (estrus) (Weiss et al., 2001). Infection of the genital tract with herpes simplex type 2 virus results in strong IgA (Th2 cell dependent) and low IgG antibody responses during estrus but strong IgG and low IgA antibody responses during diestrus (Gallichan and Rosenthal, 1996). In Thieler’s virus infections, male mice are more susceptible to encephalitis than females and pathogenesis depends upon a predominant Th1 cell response (Kim et al., 2001). Thus, sex-associated hormones have a potent impact on immunity. The precise nature of the impact on immune responses can be controversial, however. Generally, estrogens promote CD4+Th1 immune responses (Delpy et al., 2005; Karpuzoglu-Sahin, Hissong, and Ansar Ahmed, 2001; Karpuzoglu et al., 2007), but this is largely concentration dependent since high estrogen levels associated with pregnancy also suppress Th1 cell responses (Doria et al., 2006). Low-level estrogen primes T cells for T-bet activation, the transcription factor associated with IFNγ expression and characteristic for Th1 cells (Karpuzoglu et al., 2007). In contrast, androgens including progesterone, usually promote Th2 cell responses (Joachim et al., 2003; Matsuzaki et al., 2005) and apoptosis of Th1 cells (Matsuzaki et al., 2005). In picornavirus infections, however (coxsackievirus B3 and Theiler’s virus (Fuller et al., 2007; Huber and Pfaeffle, 1994; Kim et al., 2001)), androgens promote Th1 cell responses while estrogens promote Th2 cell responses. Why androgens preferentially activate Th1 cell responses in picornavirus infections is not known, but the answer might involve how sex-associated hormone affect virus replication (Fuller et al., 2007; Lyden et al., 1987) as increasing or decreasing virus load (antigen concentrations) could alter the characteristics of the anti-viral response. Studies clearly show that antigen dose can determine T cell polarization to either a Th1 (IFNγ+) or Th2 (IL-4+) phenotype (Hosken et al., 1995) with very low or very high antigen concentrations producing Th2 polarization. Evaluations of decay accelerating factor (DAF), one of the known virus receptors for coxsackieviruses (Bergelson et al., 1995), in young cycling women show that DAF levels on peripheral blood lymphocytes and infectivity/ virus production by these lymphocytes increases with estrogen levels in plasma and is highest in the follicular phase (Sartini et al., 2004).
Previously (Huber, Feldman, and Sartini, 2006), it was shown that female BALB/c mice infected in diestrus and proestrus phases of the ovarian cycle developed more myocarditis and preferential CD4+Th1 cell responses while females infected in estrus and metestrus phases developed preferential CD4+Th2 cell responses and less cardiac inflammation. The current paper provides additional information by showing that T regulatory cells are induced when females are infected in estrus and that this inhibits myocarditis induction. It is important that the basic pattern of myocarditis susceptibility is identical in BALB/c and TNF1.6 animals with suppressed disease in females infected in the estrus phase. This result indicates that hormonal impact on coxsackieviral viral disease is not mouse strain dependent. A valid question is why the current report uses TNF1.6 transgenic mice when the earlier study used BALB/c animals. Although infecting BALB/c females in different phases of the estrus phase produced consistent, reproducible and significant changes in cardiac inflammation, the differences were nonetheless modest and required actually counting individual small inflammatory loci each consisting usually of only 10–20 inflammatory cells. In contrast, differences in myocarditis severity in the transgenic animals expressing the TNFα gene under the cardiac myosin promoter are dramatic and allow evaluation by image analysis and by heart/body weights. The presence of transgenically expressed TNFα does not alter the pattern of myocarditis susceptibility but dramatically accentuates the differences in disease severity and the protective capacity of infecting females in estrus. TNF1.6 mice specifically express high TNFα concentrations in the heart but not in any other tissues or in the blood (Huber, Feldman, and Sartini, 2006; Kubota et al., 1997). TNFα is essential to myocarditis susceptibility (Lane et al., 1992; Lane et al., 1993) and is required for activation of T cells expressing the γδ T cell receptor (Huber and Sartini, 2005) which are important mediators of viral pathogenicity (Huber, Shi, and Budd, 2002). Thus, use of these transgenic mice amplifies the effects of coxsackievirus B3 infection in females making differences in myocarditis severity between groups much more dramatic than would be observed in wild-type mice where inherent levels of TNFα in the heart are negligible. It is important to note, however, that the presence of TNFα in the heart is not contributing to differences observed in infected females during the estrus cycle since neither cardiac inflammation or heart weight change in uninfected TNF1.6 mice with estrus cycle phase (Figure 1).
Whether hormonal fluctuations impact clinical myocarditis susceptibility is unclear. Men are more likely to develop clinical myocarditis than women. Susceptibility in women may increase during the peripartum period (Woodruff, 1980). This is similar to the mouse model of coxsackievirus B3 induced myocarditis where male mice are more susceptible than females but females become highly susceptible to viral myocarditis during the third trimester of pregnancy and post-partum (Huber, Job, and Auld, 1982; Lyden and Huber, 1984). It is conceivable therefore that estrogen treatments might prove beneficial in clinical myocarditis, especially in clinical disease caused by inflammatory mediators. Other studies also find that estrogen promotes T regulatory cell responses (Polanczyk et al., 2005; Tai et al., 2008) meaning that T regulatory cell responses to estrogen treatment might be a consistent phenomenon and useful in multiple immunopathogenic diseases where Th1 cell responses are involved.
MATERIALS AND METHODS
Mice
Female TNF1.6 transgenic mice were used at 6–8 weeks of age. The TNF1.6 mice have cardiac-specific TNFα over-expression and were made by microinjecting the TNFα construct into pronuclei of one cell FVB mouse embryos and implanting the embryos into pseudopregnant mice as described by Kubato et al (Kubota et al., 1997). Transgenic mice are homozygous for the FVB background. Breeding pairs were kindly supplied by Dr. A.M. Feldman (Department of Medicine, Center for Translational Medicine, Thomas Jefferson University, Philadelphia, PA) and have been maintained by heterozygous (TNF1.6/+) by wild-type (+/+) breeding.
Vaginal smears
Vaginal epithelial cells were obtained from mice and smeared onto pre-cleaned Superfrost Plus slides. Smears were done between 8 and 11 am each day. Slides were stained with Hematoxylin, modified Papanicolaou stain OG6, and Papanicolaou modified EA stain. Slides were then coverslipped and evaluated for presence of white blood cells and presence and morphology of epithelial cells to determine estrus cycle stage as previously published (Cohen et al., 2002). Mice were infected with virus within 1 min of the time the vaginal smears were obtained.
Virus infection and titration
The H3 variant of CVB3 was used (Knowlton et al., 1996). Adult mice were infected by intraperitoneal injection with 105 PFU virus in phosphate buffered saline (PBS). Animals were killed 7 days after infection. Hearts were removed, and titered using the plaque forming assay (Knowlton et al., 1996).
Hormone treatment
17-β-estradiol (Sigma Chemical Co., St. Louis, MO) was initially diluted in 100% ethanol then diluted to 400 ng/ml in PBS. Mice were injected i.p. with either 200 ng/mouse estradiol or PBS on days +1 and +3 relative to infection.
Flow cytometry
Details for intracellular cytokine staining have been published previously (Huber et al., 2001). Lymphoid cells were either isolated from peripheral blood or from the heart. Peripheral blood was taken from euthanized mice by cardiac puncture into EDTA-PBS. Lymphoid cells were isolated by centrifugation on Histopaque (Sigma). Inflammatory cells in the heart were isolated by perfusing individual hearts with PBS, mincing finely, digesting the hearts with 0.4% collagenase II (Sigma Chemical Co, St. Louis MO) and 0.25% pancreatin (Sigma), then centrifuging the cell suspension on Histopaque (Sigma) (Huber, Feldman, and Sartini, 2006). For intracellular cytokine staining, 106 cells were cultured for 4 hrs in RPMI 1640 medium containing 5% fetal bovine serum, 10 µg/ml Brefeldin A (BFA), 50 ng/ml phorbol myristate acetate (PMA), and 500 ng/ml ionomycin (Sigma). After culture, the cells were washed in PBS-1% bovine serum albumin (BSA; Sigma) containing BFA, and labeled with PerCP-Cy5.5- anti-CD4 (clone GK1.5, BD Sciences, Fair Lawn, NJ), and APC-Cy7-anti-Vγ4 (clone UC3) for 30 min, washed, then fixed in 2% paraformaldehyde for 10 min. The cells were resuspended in PBS-BSA containing 0.5% saponin, Fc Block and 1:100 dilutions of PE-anti-IFNγ (clone XMG 1.2), Alexa 647-anti-IL-4 (clone 11B11), or PE and Alexa 647-rat IgG1 (clone R3–34) and incubated for 30 min on ice. The cells were washed once in PBS-BSA-saponin and once in PBS-BSA, the resuspended in 2% paraformaldehyde. FoxP3 labeling was done using the eBioscience kit according to manufacturer’s directions. Cells were labeled with Alexa647 anti-CD4 and PerCP-Cy5.5 anti-CD25 (clone PC61) in PBS-1%BSA containing FcBlock, washed, fixed and permeabilized, then incubated with PE-anti-FoxP3 and FcBlock overnight at 4°C. Cells were washed and resuspended in PBS-2% paraformaldehyde. Additional cells were incubated with Alexa 647-anti-CD4 and PerCP-Cy5.5-anti-CD69 (clone H1.2F3), washed and resuspended in PBS-2% paraformaldehyde. Cells were analyzed using a Coulter Epics Elite flow cytometer with a single excitation wavelength (488 nm) and band filters for FITC (525 nm), PerCP-Cy5.5 (695/40 nm), and PE (575 nm). The excitation wavelength for Alexa 647 is 643 nm and a band filter of 660/20 nm. The excitation wavelength for APC-Cy7 was 595 nm and a band filter of 650 nm. The cell population was classified for cell size (forward scatter) and complexity (side scatter). At least 10,000 cells were evaluated. Positive staining was determined relative to isotype controls.
Histology
The apical half of the heart was fixed in 10% buffered formalin, sectioned, and stained with hematoxylin and eosin. Sections were scored blindly for inflammation using a 0 to 4 scale where 0 represents no inflammation and 4 represents extensive confluent inflammation.
Statistics
Statistical analysis was done by Wilcoxon Ranked Score.
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grants HL80594, HL86549 and AI45666. The authors also wish to thank Colette Charland for help with flow cytometry and Kevin Kolinich for help in preparing the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Aflatoonian R, Tuckerman E, Elliott SL, Bruce C, Aflatoonian A, Li TC, Fazeli A. Menstrual cycle-dependent changes of Toll-like receptors in endometrium. Hum Reprod. 2007;22(2):586–593. doi: 10.1093/humrep/del388. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Mohanty JG, Crowell RL, St John NF, Lublin DM, Finberg RW. Coxsackievirus B3 adapted to growth in RD cells binds to decay-accelerating factor (CD55) J Virol. 1995;69(3):1903–1906. doi: 10.1128/jvi.69.3.1903-1906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowles NE, Ni J, Kearney DL, Pauschinger M, Schultheiss HP, McCarthy R, Hare J, Bricker JT, Bowles KR, Towbin JA. Detection of viruses in myocardial tissues by polymerase chain reaction. evidence of adenovirus as a common cause of myocarditis in children and adults. J Am Coll Cardiol. 2003;42(3):466–472. doi: 10.1016/s0735-1097(03)00648-x. [DOI] [PubMed] [Google Scholar]
- Bowles NE, Towbin JA. Molecular Aspects of Myocarditis. Curr Infect Dis Rep. 2000;2(4):308–314. doi: 10.1007/s11908-000-0008-x. [DOI] [PubMed] [Google Scholar]
- Brabin L. Interactions of the female hormonal environment, susceptibility to viral infections, and disease progression. AIDS Patient Care STDS. 2002;16(5):211–221. doi: 10.1089/10872910252972267. [DOI] [PubMed] [Google Scholar]
- Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, Kovats S. Estradiol acts directly on bone marrow myeloid progenitors to differentially regulate GM-CSF or Flt3 ligand-mediated dendritic cell differentiation. J Immunol. 2008;180(2):727–738. doi: 10.4049/jimmunol.180.2.727. [DOI] [PubMed] [Google Scholar]
- Cohen PE, Zhu L, Nishimura K, Pollard JW. Colony-stimulating factor 1 regulation of neuroendocrine pathways that control gonadal function in mice. Endocrinology. 2002;143(4):1413–1422. doi: 10.1210/endo.143.4.8754. [DOI] [PubMed] [Google Scholar]
- Delpy L, Douin-Echinard V, Garidou L, Bruand C, Saoudi A, Guery JC. Estrogen enhances susceptibility to experimental autoimmune myasthenia gravis by promoting type 1-polarized immune responses. J Immunol. 2005;175(8):5050–5057. doi: 10.4049/jimmunol.175.8.5050. [DOI] [PubMed] [Google Scholar]
- Doria A, Iaccarino L, Arienti S, Ghirardello A, Zampieri S, Rampudda ME, Cutolo M, Tincani A, Todesco S. Th2 immune deviation induced by pregnancy: the two faces of autoimmune rheumatic diseases. Reprod Toxicol. 2006;22(2):234–241. doi: 10.1016/j.reprotox.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Fuller A, Yahikozawa H, So EY, Dal Canto M, Koh CS, Welsh CJ, Kim BS. Castration of male C57L/J mice increases susceptibility and estrogen treatment restores resistance to Theiler's virus-induced demyelinating disease. J Neurosci Res. 2007;85(4):871–881. doi: 10.1002/jnr.21184. [DOI] [PubMed] [Google Scholar]
- Gallichan WS, Rosenthal KL. Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female mice against herpes simplex virus type 2 infection in the genital tract. Virology. 1996;224(2):487–497. doi: 10.1006/viro.1996.0555. [DOI] [PubMed] [Google Scholar]
- Gauntt C, Huber S. Coxsackievirus experimental heart diseases. Front Biosci. 2003;8:E23–E35. doi: 10.2741/928. [DOI] [PubMed] [Google Scholar]
- Gauntt C, Sakkinen P, Rose N, Huber S. Picornaviruses: Immunopathology and autoimmunity. In: Cunningham M, Fujinami R, editors. Effects of Microbes on the Immune System. Philadelphia: Lippincott-Raven Publishers; 2000. pp. 313–329. [Google Scholar]
- Hosken NA, Shibuya K, Heath AW, Murphy KM, O'Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. J Exp Med. 1995;182(5):1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Graveline D, Born W, O'Brien R. Cytokine Production by Vgamma+ T Cell Subsets is an Important Factor Determining CD4+ Th Cell Phenotype and Susceptibility of BALB/c mice to Coxsackievirus B3-Induced Myocarditis. J Virol. 2001;75(13):5860–5868. doi: 10.1128/JVI.75.13.5860-5869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Job L, Auld K. Influence of sex hormones on coxsackie B3 virus infection in Balb/c mice. Cell Immunol. 1982;67(173–189):173–179. doi: 10.1016/0008-8749(82)90210-6. [DOI] [PubMed] [Google Scholar]
- Huber S, Kupperman J, Newell M. Hormonal regulation of CD4+ T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol. 1999;73:4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with Coxsackievirus Group B Type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S, Shi C, Budd RC. Gammadelta T cells promote a Th1 response during coxsackievirus B3 infection in vivo: role of Fas and Fas ligand. J Virol. 2002;76(13):6487–6494. doi: 10.1128/JVI.76.13.6487-6494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Born W, O'Brien R. Dual functions of murine gammadelta cells in inflammation and autoimmunity in coxsackievirus B3-induced myocarditis: role of Vgamma1+ and Vgamma4+ cells. Microbes Infect. 2005;7(3):537–543. doi: 10.1016/j.micinf.2004.12.011. [DOI] [PubMed] [Google Scholar]
- Huber SA, Feldman AM, Sartini D. Coxsackievirus B3 induces T regulatory cells, which inhibit cardiomyopathy in tumor necrosis factor-alpha transgenic mice. Circ Res. 2006;99(10):1109–1116. doi: 10.1161/01.RES.0000249405.13536.49. [DOI] [PubMed] [Google Scholar]
- Huber SA, Gauntt CJ, Sakkinen P. Enteroviruses and myocarditis: Viral pathogenesis through replication, cytokine induction and immunopathogenicity. Advances in Virus Research. 1999;51:35–80. doi: 10.1016/s0065-3527(08)60783-6. [DOI] [PubMed] [Google Scholar]
- Huber SA, Sartini D. Roles of tumor necrosis factor alpha (TNF-alpha) and the p55 TNF receptor in CD1d induction and coxsackievirus B3-induced myocarditis. J Virol. 2005;79(5):2659–2665. doi: 10.1128/JVI.79.5.2659-2665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber SA, Sartini D, Exley M. Vgamma4(+) T cells promote autoimmune CD8(+) cytolytic T-lymphocyte activation in coxsackievirus B3-induced myocarditis in mice: role for CD4(+) Th1 cells. J Virol. 2002;76(21):10785–10790. doi: 10.1128/JVI.76.21.10785-10790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A, Bebo BF, Jr, Matejuk A, Zamora A, Silverman M, Fyfe-Johnson A, Offner H. Estrogen treatment down-regulates TNF-alpha production and reduces the severity of experimental autoimmune encephalomyelitis in cytokine knockout mice. J Immunol. 2001;167(1):542–552. doi: 10.4049/jimmunol.167.1.542. [DOI] [PubMed] [Google Scholar]
- Ito A, Buenafe AC, Matejuk A, Zamora A, Silverman M, Dwyer J, Vandenbark AA, Offner H. Estrogen inhibits systemic T cell expression of TNF-alpha and recruitment of TNF-alpha(+) T cells and macrophages into the CNS of mice developing experimental encephalomyelitis. Clin Immunol. 2002;102(3):275–282. doi: 10.1006/clim.2001.5175. [DOI] [PubMed] [Google Scholar]
- Joachim R, Zenclussen AC, Polgar B, Douglas AJ, Fest S, Knackstedt M, Klapp BF, Arck PC. The progesterone derivative dydrogesterone abrogates murine stress-triggered abortion by inducing a Th2 biased local immune response. Steroids. 2003;68(10–13):931–940. doi: 10.1016/j.steroids.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Karpuzoglu-Sahin E, Hissong BD, Ansar Ahmed S. Interferon-gamma levels are upregulated by 17-beta-estradiol and diethylstilbestrol. J Reprod Immunol. 2001;52(1–2):113–127. doi: 10.1016/s0165-0378(01)00117-6. [DOI] [PubMed] [Google Scholar]
- Karpuzoglu E, Phillips RA, Gogal RM, Jr, Ansar Ahmed S. IFN-gamma-inducing transcription factor, T-bet is upregulated by estrogen in murine splenocytes: role of IL-27 but not IL-12. Mol Immunol. 2007;44(7):1808–1814. doi: 10.1016/j.molimm.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim BS, Lyman MA, Kang BS, Kang HK, Lee HG, Mohindru M, Palma JP. Pathogenesis of virus-induced immune-mediated demyelination. Immunol Res. 2001;24(2):121–130. doi: 10.1385/IR:24:2:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowlton KU, Jeon ES, Berkley N, Wessely R, Huber S. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70(11):7811–7818. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovats S, Carreras E. Regulation of dendritic cell differentiation and function by estrogen receptor ligands. Cell Immunol. 2008 doi: 10.1016/j.cellimm.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota T, McTiernan C, Frye C, Slawson S, Lemster B, Koretsky A, Demetris A, Feldman A. Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha. Circ Res. 1997;81:627–635. doi: 10.1161/01.res.81.4.627. [DOI] [PubMed] [Google Scholar]
- Lane J, Neumann D, Lafond-Walker A, Herkowitz A, Rose N. Interleukin 1 or tumor necrosis factor can promote Coxsackie B3-induced myocarditis in resistant B10.A mice. J Exp Med. 1992;175:1123–1129. doi: 10.1084/jem.175.4.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane J, Neumann D, Lanfond-Walker A, Herskowitz A, Rose N. Role of IL-1 and tumor necrosis factor in coxsackievirus-induced autoimmune myocarditis. J Immunol. 1993;151:1682–1690. [PubMed] [Google Scholar]
- Lyden D, Huber S. Aggrevation of coxsackievirus group B type 3-induced myocarditis and increase in cellular immunity to myocyte antigens in pregnant BALB/c mice and animals treated with progesterone. Cell Immunol. 1984;87:462–472. doi: 10.1016/0008-8749(84)90015-7. [DOI] [PubMed] [Google Scholar]
- Lyden D, Olszewski J, Feran M, Job L, Huber S. Coxsackievirus B3-induced myocarditis. Effects of sex steroids on viremia and infectivity of cardiocytes. Am J Pathol. 1987;126:432–438. [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki J, Tsuji T, Imazeki I, Ikeda H, Nishimura T. Immunosteroid as a regulator for Th1/Th2 balance: its possible role in autoimmune diseases. Autoimmunity. 2005;38(5):369–375. doi: 10.1080/08916930500124122. [DOI] [PubMed] [Google Scholar]
- Nie CQ, Bernard NJ, Schofield L, Hansen DS. CD4+ CD25+ regulatory T cells suppress CD4+ T-cell function and inhibit the development of Plasmodium berghei-specific TH1 responses involved in cerebral malaria pathogenesis. Infect Immun. 2007;75(5):2275–2282. doi: 10.1128/IAI.01783-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polanczyk MJ, Hopke C, Huan J, Vandenbark AA, Offner H. Enhanced FoxP3 expression and Treg cell function in pregnant and estrogen-treated mice. J Neuroimmunol. 2005;170(1–2):85–92. doi: 10.1016/j.jneuroim.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Rueckert R. Picronaviruses. In: Fields BN, Knipe DM, Howley PM, editors. Fundamental Virology. 3rd Ed. Philadelphia, PA: Lippincott-Raven; 1996. [Google Scholar]
- Sartini D, Moussawi M, Sallam R, Bernstein I, Huber S. Correlation between serum estradiol in the follicular phase of the ovarian cycle and decay acceleration factor (DAF) expression on red blood cells and coxsackiervirus B-3 induced hemagglutination in young cycling women. Am J Reprod Immunol. 2004;51(2):180–187. doi: 10.1046/j.8755-8920.2003.00142.x. [DOI] [PubMed] [Google Scholar]
- Schuster JP, Schaub GA. Trypanosoma cruzi: the development of estrus cycle and parasitemia in female mice maintained with or without male pheromones. Parasitol Res. 2001;87(12):985–993. doi: 10.1007/s004360100472. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Sartini D, Huber S. Myocarditis susceptibility in female mice depends upon ovarian cycle phase at infection. Virology. 2004;330(1):16–23. doi: 10.1016/j.virol.2004.06.051. [DOI] [PubMed] [Google Scholar]
- Sugiura K, Nishikawa M, Ishiguro K, Tajima T, Inaba M, Torii R, Hatoya S, Wijewardana V, Kumagai D, Tamada H, Sawada T, Ikehara S, Inaba T. Effect of ovarian hormones on periodical changes in immune resistance associated with estrous cycle in the beagle bitch. Immunobiology. 2004;209(8):619–627. doi: 10.1016/j.imbio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Tai P, Wang J, Jin H, Song X, Yan J, Kang Y, Zhao L, An X, Du X, Chen X, Wang S, Xia G, Wang B. Induction of regulatory T cells by physiological level estrogen. J Cell Physiol. 2008;214(2):456–464. doi: 10.1002/jcp.21221. [DOI] [PubMed] [Google Scholar]
- Weiss ML, Dobbs ME, MohanKumar PS, Chowdhury SI, Sawrey K, Guevara-Guzman R, Huang J. The estrous cycle affects pseudorabies virus (PRV) infection of the CNS. Brain Res. 2001;893(1–2):215–226. doi: 10.1016/s0006-8993(00)03320-5. [DOI] [PubMed] [Google Scholar]
- Woodruff J. Viral myocarditis. Am. J. Pathol. 1980;101:425–483. [PMC free article] [PubMed] [Google Scholar]


