Abstract
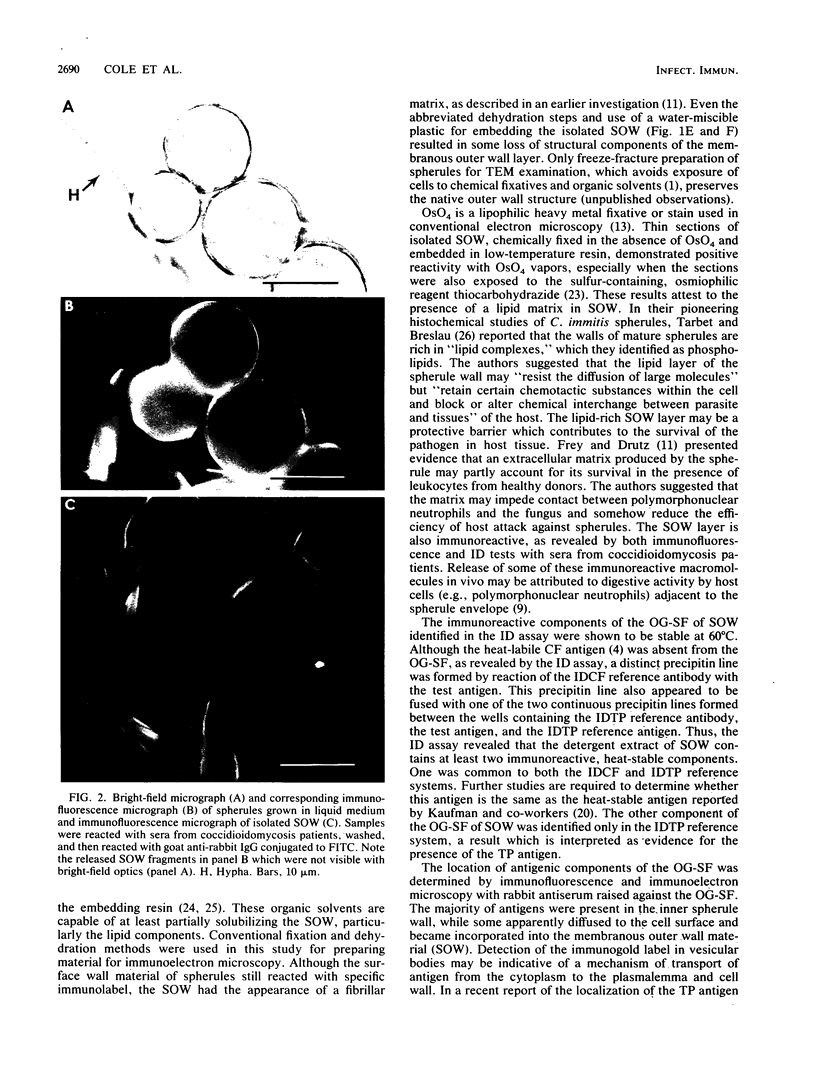
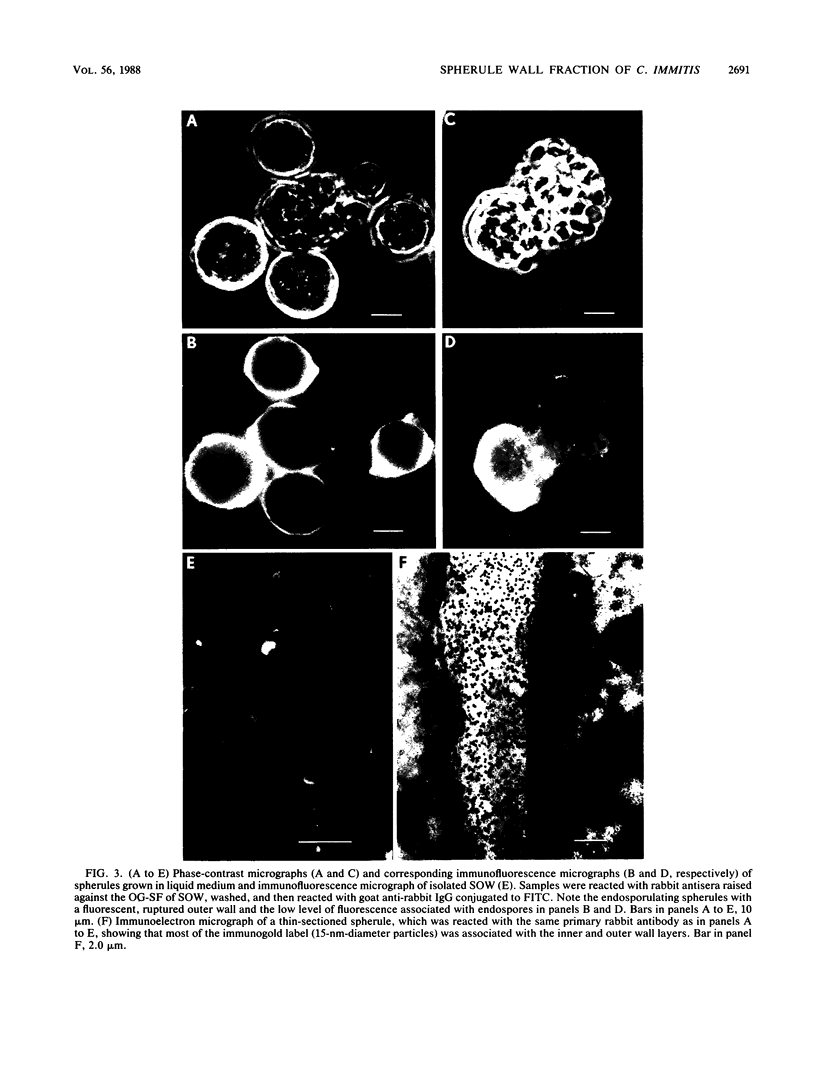
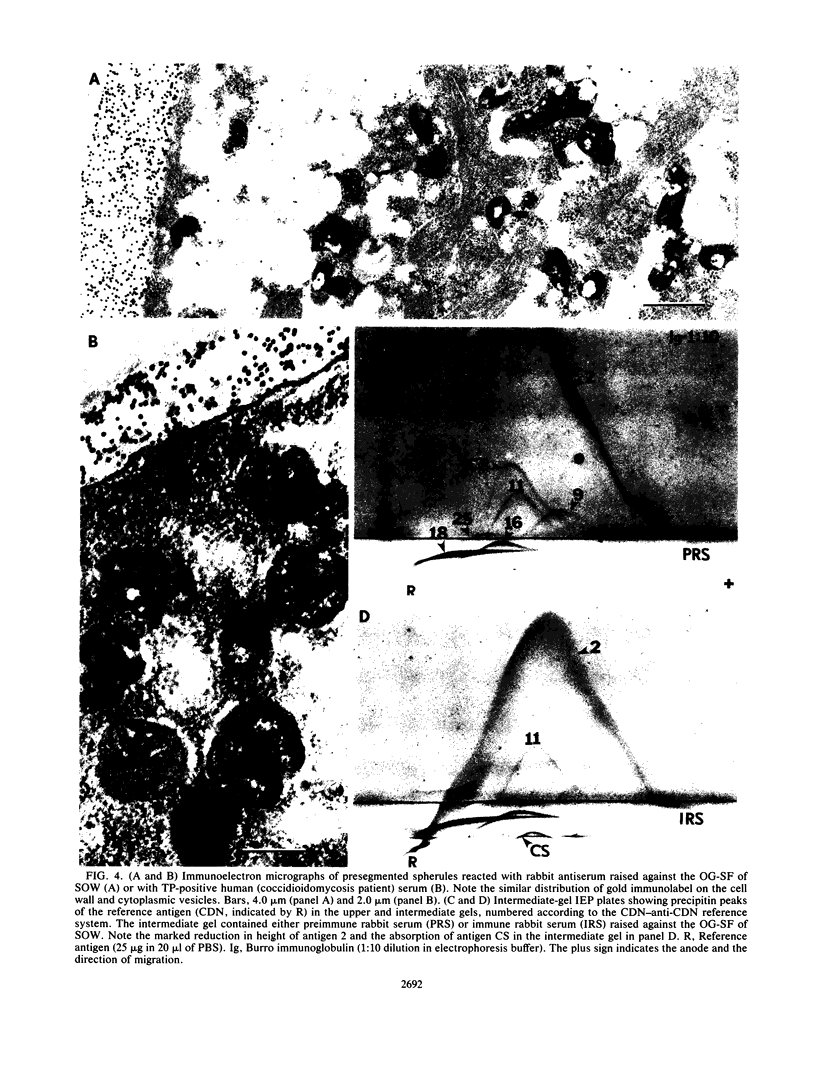
A previously undescribed, immunoreactive, membranous spherule outer wall (SOW) fraction produced by Coccidioides immitis (strains 634 and 735) grown in culture was isolated. Both this fraction and intact spherules were reactive with sera from coccidioidomycosis patients, as demonstrated by immunofluorescence microscopy. The serological activity of SOW was also demonstrated by its reactivity with human anti-C. immitis tube precipitin in a standardized immunodiffusion assay. Extraction of SOW with the nonionic detergent N-octyl-beta-D-glucopyranoside (OG) permitted the isolation of an OG-soluble fraction which was reactive in the immunodiffusion assay. Rabbit antisera raised against the OG-soluble fraction were used in immunofluorescence and immunoelectron-microscopic studies of the parasitic cycle to confirm that the immunoreactive components of the solubilized fraction of SOW were associated with the inner and outer layers of the spherule wall as well as with distinct cytoplasmic organelles observed in thin sections of spherules. The immunoreactivity of SOW with sera from patients suggested that infected individuals are exposed to this surface wall material isolated from in vitro-grown spherules.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CONVERSE J. L. Growth of spherules of Coccidioides immitis in a chemically defined liquid medium. Proc Soc Exp Biol Med. 1955 Dec;90(3):709–711. doi: 10.3181/00379727-90-22144. [DOI] [PubMed] [Google Scholar]
- Cole G. T., Kirkland T. N., Franco M., Zhu S., Yuan L., Sun S. H., Hearn V. M. Immunoreactivity of a surface wall fraction produced by spherules of Coccidioides immitis. Infect Immun. 1988 Oct;56(10):2695–2701. doi: 10.1128/iai.56.10.2695-2701.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. T., Kirkland T. N., Sun S. H. An immunoreactive, water-soluble conidial wall fraction of Coccidioides immitis. Infect Immun. 1987 Mar;55(3):657–667. doi: 10.1128/iai.55.3.657-667.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drutz D. J., Huppert M. Coccidioidomycosis: factors affecting the host-parasite interaction. J Infect Dis. 1983 Mar;147(3):372–390. doi: 10.1093/infdis/147.3.372. [DOI] [PubMed] [Google Scholar]
- Frey C. L., Drutz D. J. Influence of fungal surface components on the interaction of Coccidioides immitis with polymorphonuclear neutrophils. J Infect Dis. 1986 May;153(5):933–943. doi: 10.1093/infdis/153.5.933. [DOI] [PubMed] [Google Scholar]
- Huppert M., Adler J. P., Rice E. H., Sun S. H. Common antigens among systemic disease fungi analyzed by two-dimensional immunoelectrophoresis. Infect Immun. 1979 Feb;23(2):479–485. doi: 10.1128/iai.23.2.479-485.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huppert M., Spratt N. S., Vukovich K. R., Sun S. H., Rice E. H. Antigenic analysis of coccidioidin and spherulin determined by two-dimensional immunoelectrophoresis. Infect Immun. 1978 May;20(2):541–551. doi: 10.1128/iai.20.2.541-551.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman L., Standard P. G., Huppert M., Pappagianis D. Comparison and diagnostic value of the coccidioidin heat-stable (HS and tube precipitin) antigens in immunodiffusion. J Clin Microbiol. 1985 Oct;22(4):515–518. doi: 10.1128/jcm.22.4.515-518.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEVINE H. B. Purification of the spherule-endospore phase of Coccidioides immitis. Sabouraudia. 1961 Jun;1:112–115. doi: 10.1080/00362176285190231. [DOI] [PubMed] [Google Scholar]
- Reiss E., Huppert M., Cherniak R. Characterization of protein and mannan polysaccharide antigens of yeasts, moulds, and actinomycetes. Curr Top Med Mycol. 1985;1:172–207. doi: 10.1007/978-1-4613-9547-8_7. [DOI] [PubMed] [Google Scholar]
- Seligman A. M., Wasserkrug H. L., Hanker J. S. A new staining method (OTO) for enhancing contrast of lipid--containing membranes and droplets in osmium tetroxide--fixed tissue with osmiophilic thiocarbohydrazide(TCH). J Cell Biol. 1966 Aug;30(2):424–432. doi: 10.1083/jcb.30.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S. H., Cole G. T., Drutz D. J., Harrison J. L. Electron-microscopic observations of the Coccidioides immitis parasitic cycle in vivo. J Med Vet Mycol. 1986 Jun;24(3):183–192. [PubMed] [Google Scholar]
- Sun S. H., Sekhon S. S., Huppert M. Electron microscopic studies of saprobic and parasitic forms of Coccidioides immitis. Sabouraudia. 1979 Sep;17(3):265–273. doi: 10.1080/00362177985380391. [DOI] [PubMed] [Google Scholar]
- TARBET J. E., BRESLAU A. M. Histochemical investigation of the spherule of Coccidioides immitis in relation to host reaction. J Infect Dis. 1953 Mar-Apr;92(2):183–190. doi: 10.1093/infdis/92.2.183. [DOI] [PubMed] [Google Scholar]