Abstract
Lipid rafts is a blanket term used to describe distinct areas in the plasma membrane rich in certain lipids and proteins and which are thought to perform diverse functions. A large number of studies report on lipid rafts having a key role in receptor signalling and activation of lymphocytes. In T cells, lipid raft involvement was demonstrated in the early steps during T cell receptor (TCR) stimulation. Interestingly, recent evidence has shown that signalling in these domains differs in T cells isolated from patients with autoimmune diseases such as systemic lupus erythematosus (SLE) and rheumatoid arthritis (RA). Here, we discuss these findings and explore the potential of lipid rafts as targets for the development of a new class of agents to downmodulate immune responses and for the treatment of autoimmune diseases.
Keywords: Lipid rafts, T cell, Lupus, Autoimmunity, Review
1. Signalling by the T cell receptor (TCR)
The TCR recognizes peptides presented in the context of appropriate major histocompatibility complex (MHC) proteins expressed on the surface of antigen presenting cells (APCs). Signalling cascades that branch out of the stimulated TCR in conjunction with signals that originate from concomitantly stimulated co-receptors result in the activation of the T cell and development of effector functions [1].
The immediate outcome of TCR stimulation is tyrosine phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) present in the cytoplasmic domains of the chains (γ, δ, ɛ, and ζ) that comprise the TCR-associated CD3 complex [2,3]. ITAM phosphorylation is mediated by the Src-family tyrosine kinase Lck [4,5]. The phosphorylated TCR recruits to the plasma membrane the cytosolic ZAP-70 tyrosine kinase [6], the main substrate of which is the transmembrane adaptor LAT (linker for activation of T cells). In turn, LAT phosphorylation triggers the formation of multi-protein signalling complexes at the plasma membrane. Proteins that participate in such assemblies include other scaffold molecules such as Grb-2, SLP-76, and Gads, and enzymes like phospholipase C (PLC) γ1 and phosphoinositide 3-kinase (PI3K) [7–9]. The LAT-nucleated complexes at the cytoplasmic face of the plasma membrane control downstream signalling pathways that lead to changes in cell morphology and gene transcription.
Recognition of antigen/MHC complexes by the TCR is assisted by co-receptors, CD4 and CD8, and co-stimulatory receptors such as CD28, expressed on the surface of T cells that pair with their cognate ligands on the APC. The coordinated action of these interactions and ensuing signalling result in a stable, yet dynamic, zone of contact between the two cells which has been named the immunological synapse (IS) (reviewed in Ref. [10]). The plasticity of the IS became apparent through imaging studies showing temporal and spatial recruitment of various co-receptors and signalling molecules within subdomains of the synapse [11]. For example, in the mature IS, the central region of the synapse, where the T cell and APC membranes are in close proximity, concentrates the TCR, the CD4 and CD28 receptors, and associated signalling molecules such as Lck and protein kinase C (PKC) θ [12]. Larger and heavily glycosylated molecules such as CD44, CD45 and CD43 are excluded from the central region and preferentially occupy a peripheral area that surrounds the core of the synapse [13,14]. Lipid rafts are found to accumulate at the IS, as was reported by staining the cells with cholera toxin B subunit (CTB), which binds to Ganglioside M1 (GM1), a marker of raft membranes [15,16]. Furthermore, using the fluorescent probe Laurdan, condensation of the plasma membrane was detected at the site of the IS, suggesting accumulation of lipid rafts [17]. Interestingly, the tyrosine phosphatase CD45 has been shown to transiently move to the proximity of the TCR at the later stages during T cell/APC interaction [18]. However, initial TCR activation and early tyrosine phosphorylation precedes formation of the IS and occurs in TCR-containing microclusters which form within seconds of the initial TCR-MHC/antigen contact and contain activated Lck, ZAP-70, and LAT [19,20]. Subsequent formation of the IS not only provides the sustained signal needed for gene transcription but may also control the eventual cessation of the signal and downregulation of the immune response.
2. Lipid rafts in T cells; size matters
The term lipid raft is used to describe microdomains in the plasma membrane that are in liquid-ordered phase owing to their lipid composition which is rich in cholesterol, glycosphingolipids, and sphingomyelin [21–23]. As a result, their structural properties are different from the glycerophospholipid-rich bilayer which comprises the bulk of the plasma membrane [24]. Although it is agreed that in unstimulated cell lipid rafts are too small to resolve with the light microscope, their actual size and protein composition is an area of ongoing debate [25,26]. While some groups report a size between 50 and 200 nm [27], others have measured a much smaller size of <20 nm [28]. This is a key issue that needs to be addressed if we are to better understand their function (the reader is also referred to the accompanying reviews in this issue). For example, microdomains of a larger size will promote compartmentalization of plasma membrane proteins that have higher affinity for the liquid-ordered phase, while they will exclude proteins that have higher affinity for the liquid-disordered phase. In this scenario, some protein–protein interactions will be favoured while others will be prevented. This arrangement may change upon physiological stimulation of receptors such as the TCR. This model has been the ‘classical’ raft hypothesis. On the other hand, if lipid rafts are of smaller size (<20 nm) they will be able to accommodate only few protein molecules. Although it is difficult to see how such ‘nanodomains’ could induce any significant segmentation of the membrane, a recent computational modeling study on raft–protein dynamics indicated that small, mobile rafts can reduce the diffusion rate of associated proteins and hence increase protein–protein collisions. In contrast, proteins that are excluded from rafts did not show reduced mobility [29]. Furthermore, coalesence of these small, highly mobile, and probably short-lived, units into larger structures under certain conditions, such as receptor triggering, will enable them to adopt the properties of larger rafts as mentioned above [30]. Larger rafts are likely to be controlled by endocytosis unless stabilized by, for example, receptor stimulation [31]. Receptor-induced protein–protein interactions also have a critical role and will further facilitate aggregation and signalling [32]. In this model, any change in the status of the lymphocyte that results in higher accumulation of lipids which are constituents of raft membrane, is likely to profoundly change membrane organization by supporting formation of larger rafts. These changes may in turn influence the strength or duration of receptor signal. Thus, inappropriate changes in the size and/or structure of lipid rafts could influence their stability and may result in abnormal signalling and pathological conditions. Changes in the level of membrane cholesterol and GM1 have been reported in activated and autoimmune lymphocytes (see Fig. 1 and Section 4 below).
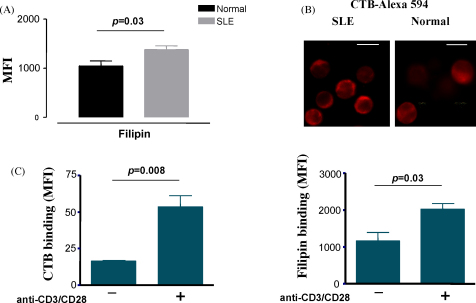
Fig. 1.
Increased levels of cholesterol and GM1 in the plasma membrane of T cells from patients with SLE and activated normal T cells. (A) Purified T cells from SLE patients and healthy controls were labelled with the cholesterol chelator filipin and analysed by flow cytometry. The results shown are the cumulative data collected from five lupus patients and five healthy volunteers. (B) SLE and normal T cells were stained with Alexa-fluor 594-conjugated cholera toxin B subunit (CTB-Alexa 594) and analysed by confocal microscopy. The images shown are adjusted to the same output intensity and are representative of samples generated from two healthy donors and two SLE patients. Bar = 10 μm. (C) Peripheral blood T cells from healthy donors were activated in vitro with a combination of anti-CD3/anti-CD28 antibodies and stained with CTB-Alexa 594 or filipin to assess the levels of GM1 and cholesterol, respectively.
3. Lipid rafts in TCR signalling
Historically, the interest of immunologists in T cell lipid rafts originated from the observation that Lck, which phosphorylates the TCR ITAMs, localizes to these domains owing to its N-terminal dual acylation [33–35]. It was shown that the ability of the kinase to support TCR signalling critically depends on its lipid modification [35,36]. Soon after, LAT was found to partition to raft domains through palmitoylation of two cysteine residues that are in juxtaposition to its transmembrane segment [7]. As with Lck, mutation of the cysteines delocalizes LAT and negates its biological function [37].
Like all the Src-family kinases, Lck activity is regulated by the phosphorylation-dephosphorylation cycle of two tyrosine (Y) residues located in the catalytic domain (Y394) and the C-terminus (Y505) of the protein [38]. Phosphorylation of Y505 by Csk (C-terminal Src kinase) [39] induces its interaction with the Src homology (SH) 2 domain of the protein causing the folding of the molecule into a ‘closed’ conformation which has low enzymatic activity [40,41]. In contrast, autophosphorylation of Y394 forces the unfolding of the protein into an ‘open’ configuration which has substantially higher activity [42].
The activity of Lck is also regulated by CD45, a receptor-type tyrosine phosphatase which dephosphorylates Y505 [40,43]. The constitutive action of CD45 maintains a pool of Lck in a primed form ready to support signalling by the TCR. The importance of CD45 has been highlighted by the absence of TCR signalling in mice and cell lines that are deficient in its expression [44,45]. It is important, however, to note that CD45 also has a negative role in signalling by dephosphorylating Lck at Y394 [46] and the CD3ζ chain [47]. Initially it was suggested that CD45 is excluded from raft domains [48]; however, subsequent reports have identified a small but detectable fraction of the phosphatase in these domains [49,50]. How the cell balances the positive and negative actions of CD45 during the early steps of TCR signalling is not yet clear. A current theory that explains its dual role suggests that, while the phosphatase constitutively maintains a pool of Lck primed for action, it is excluded from the area of TCR engagement upon stimulation of the receptor, due to its large, heavily glycosylated extracellular domain, which cannot be accommodated into the narrow space created by the close proximity of the T cell and APC surfaces [13,51]. Accumulation of lipid rafts and of Lck in these areas will shift the balance towards increased phosphorylation and signal transduction [17,52]. This theory is supported by experiments showing that CD45 chimeras in which the extracellular portion has been substituted by domains of progressively smaller size, gradually lose their ability to support TCR signalling [50]. Furthermore, it is known for many years that the various isoforms of CD45 which differ in their extracellular domain also differ in their ability to support TCR signalling [43].
A finding which at first glance seems to contradict this theory is the observation that the pool of Lck present in lipid rafts is phosphorylated on Y505 and is primarily in its ‘closed’ conformation [48,53–55]. This could be due to the presence of the PAG-Csk inhibitory complex in lipid rafts. PAG (protein associated with GEMs or otherwise known as Csk binding protein (Cbp)) is a transmembrane adaptor which, like LAT, is palmitoylated on two membrane-proximal cysteines [56,57]. This adaptor is phosphorylated by Src-family kinases and recruits Csk to lipid rafts [55,58,59]. Recruited Csk in turn phosphorylates Lck at Y505 inducing its ‘closed’ conformation [55,59]. Recently, however, the generation of PAG-deficient animals has revealed that recruitment of Csk to the membrane fraction and Lck Y505 phosphorylation is not significantly reduced in T cells, suggesting that an additional, yet unidentified, Csk anchor exists [60]. Under certain conditions, such as receptor stimulation, a tyrosine phosphatase, possibly CD45, may transiently associate with lipid rafts dephosphorylating Y505 and converting raft-associated Lck molecules into the ‘open’ structure (Fig. 2, top panel) [61]. Interestingly, chimeras of CD45 which show better localization to lipid rafts are able to support TCR signalling more efficiently [50]. Lipid rafts containing primed Lck subsequently associate with the microclusters of engaged TCRs, phosphorylating ITAMs and initiating signal transmission [62]. In this scenario formation of TCR microclusters should precede phosphorylation of the ITAMs by Lck. In support of this model Campi et al. found that, in cells treated with inhibitors of Src kinases, microclusters of engaged TCRs were able to form, although tyrosine phosphorylation was abolished [19]. Rather, microcluster formation was dependent on actin polymerization [19]. Significantly, lipid raft microdomains were found to contain actin and other cytoskeletal proteins suggesting that their movement may be regulated by reorganization of the actin cytoskeleton [63–66]. Subsequent formation of the IS will promote a wider segregation of receptors and signalling proteins excluding CD45 from the area of TCR phosphorylation allowing for sustained signalling. Therefore, compartmentalization of CD45 both inside and outside of lipid rafts could be a key issue in determining not only the threshold but also the duration of TCR activation during an immune response.
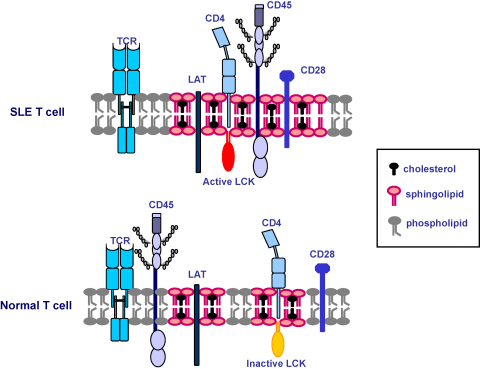
Fig. 2.
Differential association of signalling molecules to lipid raft domains in SLE may contribute to a breakdown in peripheral T cell tolerance. Higher synthesis of cholesterol and of other lipids could result in larger or more stable lipid raft domains. These changes in the plasma membrane could be the reason for the increased co-localization of CD45 phosphatase with lipid rafts and the higher Lck activity seen in SLE T cells. Enhanced protein–protein interactions and changes in enzymatic activity could lower the threshold for TCR activation.
4. Lipid raft signalling in autoimmune T cells
The homeostasis of the immune system is stringently controlled by the specificity and fidelity of lymphocyte activation. In autoimmune diseases this specificity and fidelity is compromised leading to pathology. Whether changes in the composition or structure of lipid rafts play a role in autoimmunity is an important question which has started to be addressed in the last few years. It was noted that human T cells activated in vitro via their TCR synthesize more GM1 lipid, a component of raft domains, as detected by staining with CTB [67,68] and Fig. 1C). The physiological significance of this observation is not yet clear; however, it is possible that higher GM1 and cholesterol content report on an increased portion of the plasma membrane being in the liquid-ordered phase. As a result the lateral mobility of receptors and other signalling molecules may be reduced, increasing compartmentalization of proteins at the plasma membrane. Such changes may impact on thresholds for activation of receptors and possibly of the TCR. Indeed, over-loading healthy T cells with cholesterol reduces membrane fluidity and disrupts the interaction of signalling molecules [69]. Interestingly, plasma membrane cholesterol has been linked to increased T cell immunosenescence associated with aging [70]. It is not known whether the mobility of molecules associated with glycosphingolipids such as glycosylphosphatidylinositol (GPI)-linked receptors, is altered in resting and activated cells.
Similarly, the plasma membrane of peripheral blood T cells freshly purified from patients suffering from the autoimmune disease SLE was found to contain more cholesterol and GM1, confirming that these cells have an activated phenotype ([71,72] and Fig. 1). When purified SLE T cells were cultured in vitro the levels of cholesterol and GM1 gradually decreased to that seen in naïve T cells from healthy volunteers, indicating that stimuli in the body of lupus patients activate T cells to increase synthesis of these lipids. Higher synthesis of the lipids was reinstated following TCR stimulation. Culturing the cells in the presence of serum from SLE patients did not maintain the high expression of GM1 and cholesterol, suggesting that cell to cell contacts are needed for the activation of SLE T cells in the body [71]. Relevant is the finding that dendritic cells (DCs) from patients have an activated phenotype due to the sustained action of interferon alpha (INFα) [73]. Activated DCs may be instrumental in the presentation of autoantigens contributing to the activation of T cells.
There are documented defects in the expression and function of various signalling molecules and pathways proximal to the TCR in SLE T cells [74,75]. Among these are reduced expression of the canonical ζ chain of the TCR but expression of non-conventional ζ transcripts [76], expression of the γ chain of the Fcɛ receptor [77] and reduced expression of Lck [78]. Reduction in Lck expression was more pronounced in T cells from patients with active disease and was independent of the treatment regime. Reduction of Lck expression is probably due to a combination of reduced gene transcription and increased ubiquitination/degradation of the protein [78,79]. In accordance, SLE T cells when stimulated via the TCR are poor activators of MAPK (mitogen-activated protein kinase) pathways and hypoproliferate in vitro [80,81]. Increased consumption of Lck via ubiquitination and degradation may be, at least in part, due to the sustained stimulation of lupus T cells by autoantigens, since it has been shown that sustained TCR stimulation leads to reduction in the expression of Lck [15].
Compared to healthy controls, lipid rafts from SLE T cells were found to contain higher amounts of CD45 and co-immunoprecipitation experiments revealed that a larger fraction of this pool was associated with Lck. As a consequence more Lck in lipid rafts was in the active form [71]. Therefore, an increase in the localization of CD45 into lipid rafts is a key step for Lck conversion to the active form and may be a critical step in the activation of T cells. A similar observation was made with B lymphocytes isolated from SLE patients. Here again, higher expression of GM1 was noted as assessed by CTB binding, and this was accompanied by reduced expression of Lyn, an Src-family kinase responsible for the phosphorylation of the ITAMs in the B cell receptor (BCR) chains [82]. As in T cells, there was an increase in the level of CD45 present in lipid rafts in SLE B cells. Upon activation, CD45 recruitment to the site of activated BCRs was more prolonged in SLE B cells compared to cells from healthy volunteers, as was recruitment of Lyn [83]. Like in T cells, these observations may indicate that B cells in SLE patients are responding to a chronic stimulation by autoantigens.
One way of addressing the importance of higher cholesterol synthesis in TCR signalling is to treat cells with statins. These are small molecule inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, the rate-limiting enzyme in the biosynthetic pathway of cholesterol. Treatment of SLE T cells with artorvastatin inhibited the co-localization of CD45 and Lck in lipid rafts resulting in the reduction of active Lck [84]. This correlated with restoration of Lck expression back to normal levels and ERK activation following TCR triggering [84]. Collectively these observations suggest that increased synthesis of cholesterol is associated with protein–protein interactions and signalling events that determine the activation status of the lymphocyte. Therefore, pharmacological modification of the structure and/or composition of lipid raft domains may represent a new method to downregulate immune responses in pathological conditions. Statins are already under investigation as potential immuno-modulatory drugs in a range of inflammatory and autoimmune diseases.
A second chronic inflammatory autoimmune disease where T cells may play a role in the pathogenesis is rheumatoid arthritis (RA). In this case, T cells that are purified from the synovial fluid (SF) of inflamed joints, in contrast to T cells from the periphery, were shown to have an activated phenotype, yet they were hyporesponsive to TCR stimuli and proliferated poorly [85,86]. This hyporesponsiveness may be the result of chronic oxidative stress characteristic of the microenvironment of inflamed joints. In T cells, oxidative stress was shown to result in the displacement of LAT from the membrane [87]. This was due to a reduction in the levels of the anti-oxidant glutathione (GSH). Addition of N-acetyl-l-cysteine restored LAT localization to the membrane and TCR signalling [87,88]. Furthermore, reactive oxygen species (ROS)-mediated modification of Lck structure has been reported in SF, but not peripheral blood T cells, from RA patients [89]. Therefore, under certain conditions ROS-mediated modification of cysteine residues, which are targets of S-acylation, could delocalize proteins from lipid rafts and result in inhibition of activation. This is highlighted by reports showing that mutant mice with reduced capacity to produce ROS due to a polymorphism in the neutrophil cytosolic factor 1 (Ncf1) are more prone to severe arthritis [90]. Furthermore, in T cells, ROS production regulates surface redox levels to suppress autoreactivity and development of arthritis [91]. A similar situation has been observed with tumour infiltrating T cells (TILs) which, like SF T cells, are in a high oxidative stress microenvironment and are defective in their ability to fully mobilize signalling pathways following TCR stimulation.
Taken together, these observations underline the importance of lipid raft localization of proteins such as Lck, LAT and CD45, as central to TCR signalling. Increased synthesis of cholesterol and of other lipids may alter the structure of the plasma membrane of lymphocytes in a way that favours protein interactions and signalling. On the other hand, under certain conditions modification of critical residues in raft-associated proteins will disrupt their localization and inhibit cell activation. In both cases these changes profoundly impact on the function of lymphocytes by either supporting autoimmunity or failing to mount a response, respectively.
5. Conclusions and perspectives
A supposition of the lipid raft theory is that cholesterol and certain other lipids play an elementary role in the formation of membrane domains. Results from initial studies investigating in vitro activated lymphocytes or lymphocytes isolated from patients with autoimmune diseases show that the levels of cholesterol and GM1 (and possibly of other lipids) are increased compared to healthy cells. It is feasible that changes in lipid composition modify membrane organization possibly by increasing the abundance or the size of lipid raft domains. Alteration of the plasma membrane may be linked to the changes in localization and function of signalling proteins seen in autoimmune lymphocytes, although modulation of other cellular functions such as trafficking, internalization, membrane fusion and others will almost certainly occur, which in turn will have further impact on signalling. This in itself may not be sufficient to start an autoimmune response; however, in conjunction with other predisposing factors it could facilitate initiation of autoimmunity. If this is a correct assumption then it raises the possibility that lipid rafts may be targets for the development of pharmaceuticals to control aberrant immune responses. Statins are obvious candidates for such intervention and their efficacy in autoimmune diseases is currently under investigation [92,93]. On the other hand, sterol-chelating agents, such as the antibiotic nystatin which is used as an anti-fungal treatment, may have the opposite effect by assisting in the activation of lymphocytes [94,95], although detailed studies on its mechanism of action on lymphocytes are limited. Nevertheless, the prospect of lipid raft research moving closer to clinical application is an exciting new development in the field, although remaining controversies and potential new hurdles should not be underestimated.
Acknowledgements
The authors are supported by the Arthritis Research Campaign (ARC) UK, project grants 16018 and 13967. We thank David Isenberg, Michael Ehrenstein, and Claudia Mauri for their continuous support.
Contributor Information
Elizabeth C. Jury, Email: e.jury@ucl.ac.uk.
Panagiotis S. Kabouridis, Email: p.s.kabouridis@qmul.ac.uk.
References
- 1.Cantrell D. T cell antigen receptor signal transduction pathways. Annu Rev Immunol. 1996;14:259–274. doi: 10.1146/annurev.immunol.14.1.259. [DOI] [PubMed] [Google Scholar]
- 2.Irving B.A., Chan A.C., Weiss A. Functional characterization of a signal-transducing motif present in the T cell antigen receptor zeta chain. J Exp Med. 1993;177:1093–1103. doi: 10.1084/jem.177.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latour S., Veillette A. Proximal protein tyrosine kinases in immunoreceptor signaling. Curr Opin Immunol. 2001;13:299–306. doi: 10.1016/s0952-7915(00)00219-3. [DOI] [PubMed] [Google Scholar]
- 4.Molina T.J., Kishihara K., Siderovski D.P., van Ewijk W., Narendran A., Timms E. Profound block in thymocyte development in mice lacking p56lck. Nature. 1992;357:161–164. doi: 10.1038/357161a0. [DOI] [PubMed] [Google Scholar]
- 5.Straus D.B., Weiss A. Genetic evidence for the involvement of the lck tyrosine kinase in signal transduction through the T cell antigen receptor. Cell. 1992;70:585–593. doi: 10.1016/0092-8674(92)90428-f. [DOI] [PubMed] [Google Scholar]
- 6.Chan A.C., Iwashima M., Turck C.W., Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCR zeta chain. Cell. 1992;71:649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W., Sloan-Lancaster J., Kitchen J., Trible R.P., Samelson L.E. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92:83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 8.Finco T.S., Kadlecek T., Zhang W., Samelson L.E., Weiss A. LAT is required for TCR-mediated activation of PLCg1 and the Ras pathway. Immunity. 1998;9:617–626. doi: 10.1016/s1074-7613(00)80659-7. [DOI] [PubMed] [Google Scholar]
- 9.Harder T., Kuhn M. Selective accumulation of raft-associated membrane protein LAT in T cell receptor signaling assemblies. J Cell Biol. 2000;151:199–208. doi: 10.1083/jcb.151.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bromley S.K., Burack W.R., Johnson K.G., Somersalo K., Sims T.N., Sumen C. The immunological synapse. Annu Rev Immunol. 2001;19:375–396. doi: 10.1146/annurev.immunol.19.1.375. [DOI] [PubMed] [Google Scholar]
- 11.Depoil D., Zaru R., Guiraud M., Chauveau A., Harriague J., Bismuth G. Immunological synapses are versatile structures enabling selective T cell polarization. Immunity. 2005;22:185–194. doi: 10.1016/j.immuni.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Holdorf A.D., Lee K.H., Burack W.R., Allen P.M., Shaw A.S. Regulation of Lck activity by CD4 and CD28 in the immunological synapse. Nat Immunol. 2002;3:259–264. doi: 10.1038/ni761. [DOI] [PubMed] [Google Scholar]
- 13.Freiberg B.A., Kupfer H., Maslanik W., Delli J., Kappler J., Zaller D.M. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–917. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 14.Leupin O., Zaru R., Laroche T., Muller S., Valitutti S. Exclusion of CD45 from the T-cell receptor signaling area in antigen-stimulated T lymphocytes. Curr Biol. 2000;10:277–280. doi: 10.1016/s0960-9822(00)00362-6. [DOI] [PubMed] [Google Scholar]
- 15.Viola A., Schroeder S., Sakakibara Y., Lanzavecchia A. T lymphocyte costimulation mediated by reorganization of membrane microdomains. Science. 1999;283:680–682. doi: 10.1126/science.283.5402.680. [DOI] [PubMed] [Google Scholar]
- 16.Janes P.W., Ley S.C., Magee A.I. Aggregation of lipid rafts accompanies signaling via the T Cell antigen receptor. J Cell Biol. 1999;147:447–461. doi: 10.1083/jcb.147.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaus K., Chklovskaia E., Fazekas de St Groth B., Jessup W., Harder T. Condensation of the plasma membrane at the site of T lymphocyte activation. J Cell Biol. 2005;171:121–131. doi: 10.1083/jcb.200505047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson K.G., Bromley S.K., Dustin M.L., Thomas M.L. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc Natl Acad Sci USA. 2000;97:10138–10143. doi: 10.1073/pnas.97.18.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Campi G., Varma R., Dustin M.L. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–1036. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yokosuka T., Sakata-Sogawa K., Kobayashi W., Hiroshima M., Hashimoto-Tane A., Tokunaga M. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–1262. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 21.Edidin M. Lipid microdomains in cell surface membranes. Curr Opin Struct Biol. 1997;7:528–532. doi: 10.1016/s0959-440x(97)80117-0. [DOI] [PubMed] [Google Scholar]
- 22.Brown D.A., London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- 23.Simons K., Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- 24.Simons K., Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- 25.Munro S. Lipid rafts: elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 26.Shaw A.S. Lipid rafts: now you see them, now you don’t. Nat Immunol. 2006;7:1139–1142. doi: 10.1038/ni1405. [DOI] [PubMed] [Google Scholar]
- 27.Zacharias D.A., Violin J.D., Newton A.C., Tsien R.Y. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 28.Pralle A., Keller P., Florin E.L., Simons K., Horber J.K. Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J Cell Biol. 2000;148:997–1008. doi: 10.1083/jcb.148.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolau D.V., Jr., Burrage K., Parton R.G., Hancock J.F. Identifying optimal lipid raft characteristics required to promote nanoscale protein–protein interactions on the plasma membrane. Mol Cell Biol. 2006;26:313–323. doi: 10.1128/MCB.26.1.313-323.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hancock J.F. Lipid rafts: contentious only from simplistic standpoints. Nat Rev Mol Cell Biol. 2006;7:456–462. doi: 10.1038/nrm1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tanimura N., Nagafuku M., Minaki Y., Umeda Y., Hayashi F., Sakakura J. Dynamic changes in the mobility of LAT in aggregated lipid rafts upon T cell activation. J Cell Biol. 2003;160:125–135. doi: 10.1083/jcb.200207096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douglass A.D., Vale R.D. Single-molecule microscopy reveals plasma membrane microdomains created by protein–protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–950. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shenoy-Scaria A.M., Gauen L.K.T., Kwong J., Shaw A.S., Lublin D.M. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosyl-phosphatidylinositol-anchored proteins. Mol Cell Biol. 1993;13:6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rodgers W., Crise B., Rose J.K. Signals determining protein tyrosine kinase and glycosyl-phosphatidylinositol-anchored protein targeting to a glycolipid-enriched membrane fraction. Mol Cell Biol. 1994;14:5384–5391. doi: 10.1128/mcb.14.8.5384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kabouridis P.S., Magee A.I., Ley S.C. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hawash I.Y., Hu X.E., Adal A., Cassady J.M., Geahlen R.L., Harrison M.L. The oxygen-substituted palmitic acid analogue, 13-oxypalmitic acid, inhibits Lck localization to lipid rafts and T cell signaling. Biochim Biophys Acta. 2002;1589:140–150. doi: 10.1016/s0167-4889(02)00165-9. [DOI] [PubMed] [Google Scholar]
- 37.Zhang W., Trible R.P., Samelson L.E. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 38.Reynolds P.J., Hurley T.R., Sefton B.M. Functional analysis of the SH2 and SH3 domains of the lck tyrosine protein kinase. Oncogene. 1992;7:1949–1955. [PubMed] [Google Scholar]
- 39.Bergman M., Mustelin T., Oetken C., Partanen J., Flint N.A., Amrein K.E. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J. 1992;11:2919–2924. doi: 10.1002/j.1460-2075.1992.tb05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mustelin T., Altman A. Dephosphorylation and activation of the T cell tyrosine kinase pp56lck by the leukocyte common antigen (CD45) Oncogene. 1990;5:809–813. [PubMed] [Google Scholar]
- 41.Xu W., Doshi A., Lei M., Eck M.J., Harrison S.C. Crystal structures of c-Src reveal features of its autoinhibitory mechanism. Mol Cell. 1999;3:629–638. doi: 10.1016/s1097-2765(00)80356-1. [DOI] [PubMed] [Google Scholar]
- 42.Yamaguchi H., Hendrickson W.A. Structural basis for activation of human lymphocyte kinase Lck upon tyrosine phosphorylation. Nature. 1996;384:484–489. doi: 10.1038/384484a0. [DOI] [PubMed] [Google Scholar]
- 43.Penninger J.M., Irie-Sasaki J., Sasaki T., Oliveira-dos-Santos A.J. CD45: new jobs for an old acquaintance. Nat Immunol. 2001;2:389–396. doi: 10.1038/87687. [DOI] [PubMed] [Google Scholar]
- 44.Kishihara K., Penninger J., Wallace V.A., Kundig T.M., Kawai K., Wakeham A. Normal B lymphocyte development but impaired T cell maturation in CD45-exon6 protein tyrosine phosphatase-deficient mice. Cell. 1993;74:143–156. doi: 10.1016/0092-8674(93)90302-7. [DOI] [PubMed] [Google Scholar]
- 45.Koretzky G.A., Picus J., Thomas M.L., Weiss A. Tyrosine phosphatase CD45 is essential for coupling T-cell antigen receptor to the phosphatidyinositol pathway. Nature. 1990;346:66–68. doi: 10.1038/346066a0. [DOI] [PubMed] [Google Scholar]
- 46.D’Oro U., Ashwell J.D. Cutting edge: the CD45 tyrosine phosphatase is an inhibitor of Lck activity in thymocytes. J Immunol. 1999;162:1879–1883. [PubMed] [Google Scholar]
- 47.Furukawa T., Itoh M., Krueger N.X., Streuli M., Saito H. Specific interaction of the CD45 protein-tyrosine phosphatase with tyrosine-phosphorylated CD3 zeta chain. Proc Natl Acad Sci USA. 1994;91:10928–10932. doi: 10.1073/pnas.91.23.10928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rodgers W., Rose J.K. Exclusion of CD45 inhibits activity of p56lck associated with glycolipid-enriched membrane domains. J Cell Biol. 1996;135:1515–1523. doi: 10.1083/jcb.135.6.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Edmonds S.D., Ostergaard H.L. Dynamic association of CD45 with detergent-insoluble microdomains in T lymphocytes. J Immunol. 2002;169:5036–5042. doi: 10.4049/jimmunol.169.9.5036. [DOI] [PubMed] [Google Scholar]
- 50.Irles C., Symons A., Michel F., Bakker T.R., van der Merwe P.A., Acuto O. CD45 ectodomain controls interaction with GEMs and Lck activity for optimal TCR signaling. Nat Immunol. 2003;4:189–197. doi: 10.1038/ni877. [DOI] [PubMed] [Google Scholar]
- 51.van der Merwe P.A. The TCR triggering puzzle. Immunity. 2001;14:665–668. doi: 10.1016/s1074-7613(01)00155-8. [DOI] [PubMed] [Google Scholar]
- 52.Burack W.R., Lee K.H., Holdorf A.D., Dustin M.L., Shaw A.S. Cutting edge: quantitative imaging of raft accumulation in the immunological synapse. J Immunol. 2002;169:2837–2841. doi: 10.4049/jimmunol.169.6.2837. [DOI] [PubMed] [Google Scholar]
- 53.Kabouridis P.S., Janzen J., Magee A.I., Ley S.C. Cholesterol depletion disrupts lipid rafts and modulates the activity of multiple signaling pathways in T lymphocytes. Eur J Immunol. 2000;30:954–963. doi: 10.1002/1521-4141(200003)30:3<954::AID-IMMU954>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 54.Kosugi A., Sakakura J., Yasuda K., Ogata M., Hamaoka T. Involvement of SHP-1 tyrosine phosphatase in TCR-mediated signaling pathways in lipid rafts. Immunity. 2001;14:669–680. doi: 10.1016/s1074-7613(01)00146-7. [DOI] [PubMed] [Google Scholar]
- 55.Kabouridis P.S. Selective interaction of LAT (linker of activated T cells) with the open-active form of Lck in lipid rafts reveals a new mechanism for the regulation of Lck in T cells. Biochem J. 2003;371:907–915. doi: 10.1042/BJ20021578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brdicka T., Pavlistova D., Leo A., Bruyns E., Korinek V., Angelisova P. Phosphoprotein associated with glycosphingolipid-enriched microdomains (PAG), a novel ubiquitously expressed transmembrane adaptor protein, binds the protein tyrosine kinase csk and is involved in regulation of T cell activation. J Exp Med. 2000;191:1591–1604. doi: 10.1084/jem.191.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kawabuchi M., Satomi Y., Takao T., Shimonishi Y., Nada S., Nagai K. Transmembrane phosphoprotein Cbp regulates the activities of Src-family tyrosine kinases. Nature. 2000;404:999–1003. doi: 10.1038/35010121. [DOI] [PubMed] [Google Scholar]
- 58.Davidson D., Bakinowski M., Thomas M.L., Horejsi V., Veillette A. Phosphorylation-dependent regulation of T-cell activation by PAG/Cbp, a lipid raft-associated transmembrane adaptor. Mol Cell Biol. 2003;23:2017–2028. doi: 10.1128/MCB.23.6.2017-2028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yasuda K., Nagafuku M., Shima T., Okada M., Yagi T., Yamada T. Fyn is essential for tyrosine phosphorylation of Csk-binding protein/phosphoprotein associated with glycolipid-enriched microdomains in lipid rafts in resting T cells. J Immunol. 2002;169:2813–2817. doi: 10.4049/jimmunol.169.6.2813. [DOI] [PubMed] [Google Scholar]
- 60.Dobenecker M.W., Schmedt C., Okada M., Tarakhovsky A. The ubiquitously expressed Csk adaptor protein Cbp is dispensable for embryogenesis and T-cell development and function. Mol Cell Biol. 2005;25:10533–10542. doi: 10.1128/MCB.25.23.10533-10542.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torgersen K.M., Vang T., Abrahamsen H., Yaqub S., Horejsi V., Schraven B. Release from tonic inhibition of T cell activation through transient displacement of C-terminal Src kinase (Csk) from lipid rafts. J Biol Chem. 2001;276:29313–29318. doi: 10.1074/jbc.C100014200. [DOI] [PubMed] [Google Scholar]
- 62.Ike H., Kosugi A., Kato A., Iino R., Hirano H., Fujiwara T. Mechanism of Lck recruitment to the T-cell receptor cluster as studied by single-molecule-fluorescence video imaging. Chemphyschem. 2003;4:620–626. doi: 10.1002/cphc.200300670. [DOI] [PubMed] [Google Scholar]
- 63.von Haller P.D., Donohoe S., Goodlett D.R., Aebersold R., Watts J.D. Mass spectrometric characterization of proteins extracted from Jurkat T cell detergent-resistant membrane domains. Proteomics. 2001;1:1010–1021. doi: 10.1002/1615-9861(200108)1:8<1010::AID-PROT1010>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 64.Foster L.J., De Hoog C.L., Mann M. Unbiased quantitative proteomics of lipid rafts reveals high specificity for signaling factors. Proc Natl Acad Sci USA. 2003;100:5813–5818. doi: 10.1073/pnas.0631608100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bini L., Pacini S., Liberatori S., Valensin S., Pellegrini M., Raggiaschi R. Extensive temporally regulated reorganization of the lipid raft proteome following T-cell antigen receptor triggering. Biochem J. 2003;369:301–309. doi: 10.1042/BJ20020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Razzaq T.M., Ozegbe P., Jury E.C., Sembi P., Blackwell N.M., Kabouridis P.S. Regulation of T-cell receptor signalling by membrane microdomains. Immunology. 2004;113:413–426. doi: 10.1111/j.1365-2567.2004.01998.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tuosto L., Parolini I., Schroder S., Sargiacomo M., Lanzavecchia A., Viola A. Organization of plasma membrane functional rafts upon T cell activation. Eur J Immunol. 2001;31:345–349. doi: 10.1002/1521-4141(200102)31:2<345::aid-immu345>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 68.Tani-ichi S., Maruyama K., Kondo N., Nagafuku M., Kabayama K., Inokuchi J. Structure and function of lipid rafts in human activated T cells. Int Immunol. 2005;17:749–758. doi: 10.1093/intimm/dxh257. [DOI] [PubMed] [Google Scholar]
- 69.Nguyen D.H., Espinoza J.C., Taub D.D. Cellular cholesterol enrichment impairs T cell activation and chemotaxis. Mech Ageing Dev. 2004;125:641–650. doi: 10.1016/j.mad.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 70.Larbi A., Douziech N., Khalil A., Dupuis G., Gherairi S., Guerard K.P. Effects of methyl-beta-cyclodextrin on T lymphocytes lipid rafts with aging. Exp Gerontol. 2004;39:551–558. doi: 10.1016/j.exger.2003.10.031. [DOI] [PubMed] [Google Scholar]
- 71.Jury E.C., Kabouridis P.S., Flores-Borja F., Mageed R.A., Isenberg D.A. Altered lipid raft-associated signaling and ganglioside expression in T lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 2004;113:1176–1187. doi: 10.1172/JCI20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krishnan S., Nambiar M.P., Warke V.G., Fisher C.U., Mitchell J., Delaney N. Alterations in lipid raft composition and dynamics contribute to abnormal T cell responses in systemic lupus erythematosus. J Immunol. 2004;172:7821–7831. doi: 10.4049/jimmunol.172.12.7821. [DOI] [PubMed] [Google Scholar]
- 73.Blanco P., Palucka A.K., Gill M., Pascual V., Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 74.Kammer G.M., Perl A., Richardson B.C., Tsokos G.C. Abnormal T cell signal transduction in systemic lupus erythematosus. Arthritis Rheum. 2002;46:1139–1154. doi: 10.1002/art.10192. [DOI] [PubMed] [Google Scholar]
- 75.Tsokos G.C., Nambiar M.P., Tenbrock K., Juang Y.T. Rewiring the T-cell: signaling defects and novel prospects for the treatment of SLE. Trends Immunol. 2003;24:259–263. doi: 10.1016/s1471-4906(03)00100-5. [DOI] [PubMed] [Google Scholar]
- 76.Nambiar M.P., Enyedy E.J., Fisher C.U., Krishnan S., Warke V.G., Gilliland W.R. Abnormal expression of various molecular forms and distribution of T cell receptor zeta chain in patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:163–174. doi: 10.1002/1529-0131(200201)46:1<163::AID-ART10065>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 77.Enyedy E.J., Nambiar M.P., Liossis S.N., Dennis G., Kammer G.M., Tsokos G.C. Fc epsilon receptor type I gamma chain replaces the deficient T cell receptor zeta chain in T cells of patients with systemic lupus erythematosus. Arthritis Rheum. 2001;44:1114–1121. doi: 10.1002/1529-0131(200105)44:5<1114::AID-ANR192>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 78.Jury E.C., Kabouridis P.S., Abba A., Mageed R.A., Isenberg D.A. Increased ubiquitination and reduced expression of LCK in T lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2003;48:1343–1354. doi: 10.1002/art.10978. [DOI] [PubMed] [Google Scholar]
- 79.Baechler E.C., Batliwalla F.M., Karypis G., Gaffney P.M., Ortmann W.A., Espe K.J. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc Natl Acad Sci USA. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cedeno S., Cifarelli D.F., Blasini A.M., Paris M., Placeres F., Alonso G. Defective activity of ERK-1 and ERK-2 mitogen-activated protein kinases in peripheral blood T lymphocytes from patients with systemic lupus erythematosus: potential role of altered coupling of Ras guanine nucleotide exchange factor hSos to adapter protein Grb2 in lupus T cells. Clin Immunol. 2003;106:41–49. doi: 10.1016/s1521-6616(02)00052-9. [DOI] [PubMed] [Google Scholar]
- 81.Alcocer-Varela J., Alarcon-Segovia D. Decreased production of and response to interleukin-2 by cultured lymphocytes from patients with systemic lupus erythematosus. J Clin Invest. 1982;69:1388–1392. doi: 10.1172/JCI110579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Flores-Borja F., Kabouridis P.S., Jury E.C., Isenberg D.A., Mageed R.A. Decreased Lyn expression and translocation to lipid raft signaling domains in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2005;52:3955–3965. doi: 10.1002/art.21416. [DOI] [PubMed] [Google Scholar]
- 83.Flores-Borja F., Kabouridis P.S., Jury E.C., Isenberg D.A., Mageed R.A. Altered lipid raft-associated proximal signaling and translocation of CD45 tyrosine phosphatase in B lymphocytes from patients with systemic lupus erythematosus. Arthritis Rheum. 2007;56:291–302. doi: 10.1002/art.22309. [DOI] [PubMed] [Google Scholar]
- 84.Jury E.C., Isenberg D.A., Mauri C., Ehrenstein M.R. Atorvastatin restores lck expression and lipid raft-associated signaling in T Cells from patients with systemic lupus erythematosus. J Immonol. 2006;177 doi: 10.4049/jimmunol.177.10.7416. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi H., Soderstrom K., Nilsson E., Kiessling R., Patarroyo M. Integrins and other adhesion molecules on lymphocytes from synovial fluid and peripheral blood of rheumatoid arthritis patients. Eur J Immunol. 1992;22:2879–2885. doi: 10.1002/eji.1830221119. [DOI] [PubMed] [Google Scholar]
- 86.al-Janadi N., al-Dalaan A., al-Balla S., Raziuddin S. CD4+ T cell inducible immunoregulatory cytokine response in rheumatoid arthritis. J Rheumatol. 1996;23:809–814. [PubMed] [Google Scholar]
- 87.Gringhuis S.I., Leow A., Papendrecht-Van Der Voort E.A., Remans P.H., Breedveld F.C., Verweij C.L. Displacement of linker for activation of T cells from the plasma membrane due to redox balance alterations results in hyporesponsiveness of synovial fluid T lymphocytes in rheumatoid arthritis. J Immunol. 2000;164:2170–2179. doi: 10.4049/jimmunol.164.4.2170. [DOI] [PubMed] [Google Scholar]
- 88.Gringhuis S.I., Papendrecht-van der Voort E.A., Leow A., Nivine Levarht E.W., Breedveld F.C., Verweij C.L. Effect of redox balance alterations on cellular localization of LAT and downstream T-cell receptor signaling pathways. Mol Cell Biol. 2002;22:400–411. doi: 10.1128/MCB.22.2.400-411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Romagnoli P., Strahan D., Pelosi M., Cantagrel A., van Meerwijk J.P. A potential role for protein tyrosine kinase p56(lck) in rheumatoid arthritis synovial fluid T lymphocyte hyporesponsiveness. Int Immunol. 2001;13:305–312. doi: 10.1093/intimm/13.3.305. [DOI] [PubMed] [Google Scholar]
- 90.Hultqvist M., Olofsson P., Holmberg J., Backstrom B.T., Tordsson J., Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci USA. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gelderman K.A., Hultqvist M., Holmberg J., Olofsson P., Holmdahl R. T cell surface redox levels determine T cell reactivity and arthritis susceptibility. Proc Natl Acad Sci USA. 2006;103:12831–12836. doi: 10.1073/pnas.0604571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Greenwood J., Steinman L., Zamvil S.S. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol. 2006;6:358–370. doi: 10.1038/nri1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ehrenstein M.R., Jury E.C., Mauri C. Statins for atherosclerosis—as good as it gets? N Engl J Med. 2005;352:73–75. doi: 10.1056/NEJMe048326. [DOI] [PubMed] [Google Scholar]
- 94.Hammarstrom L., Smith E. Mitogenic properties of polyene antibiotics for murine B cells. Scand J Immunol. 1976;5:37–43. doi: 10.1111/j.1365-3083.1976.tb02990.x. [DOI] [PubMed] [Google Scholar]
- 95.Klein D.L., Aszalos A., Pearson J.W. Macrophage cytostasis and T and B cell blastogenic transformation in mice treated with nystatin. J Immunopharmacol. 1980;2:367–380. doi: 10.3109/08923978009046467. [DOI] [PubMed] [Google Scholar]