Abstract
Objectives
Sedentary lifestyle increases the risk of cardiovascular disease and diabetes. Vascular dysfunction contributes to atherogenesis and has been linked to insulin resistance.
Methods and Results
We measured insulin sensitivity by glucose tolerance test and vascular function by ultrasound and venous occlusion plethysmography in 20 healthy subjects (14 men, 6 women) at baseline and during 5 days of bed rest. Bed rest led to a 67% increase in the insulin response to glucose loading (P<0.001) suggesting increased insulin resistance and produced increases in total cholesterol and triglycerides. Bed rest led to decreased reactive hyperemia in the forearm (1317±404 to 1112±260 mL/min, P=0.01) and the calf (28.5±7.0 to 22.2±8.7 mL/min/dL, P=0.003) indicating impaired microvascular function. Bed rest decreased brachial artery diameter and increased systolic blood pressure suggesting increased basal arterial tone. There were no changes in circulating inflammatory markers arguing against systemic inflammation as a mechanism for vascular dysfunction in this setting.
Conclusions
Physical inactivity was associated with the development of insulin resistance, dyslipidemia, increased blood pressure, and impaired microvascular function in healthy volunteers. Our findings may provide insight into the pathogenesis of vascular disease in sedentary individuals and emphasize that even short-term physical inactivity may have adverse metabolic and vascular consequences.
Keywords: insulin resistance, sedentary lifestyle, obesity, endothelium, blood pressure, reactive hyperemia
Sedentary lifestyle increases the risk of developing cardiovascular disease and diabetes.1,2 Public health investigations indicate that more than 200 000 cardiovascular deaths per year can be attributed to physical inactivity in the United States.3 Despite compelling evidence that physical inactivity is detrimental to cardiovascular health, over one quarter of all Americans engage in no leisure time physical activity.4 The pathways leading from a sedentary lifestyle to insulin resistance and atherosclerosis are incompletely understood.
Dysfunction of the vascular endothelium contributes to atherogenesis5 and has been linked to sedentary lifestyle. In cross-sectional studies, sedentary individuals have impaired endothelial vasomotor function compared with those who are physically active.6 Sedentary individuals also display impaired reactive hyperemia,7 which is the increase in blood flow that occurs after transient ischemia and is a complex response that reflects both endothelium-dependent and endothelium-independent dilation of resistance vessels.8 States of insulin resistance, including type 2 diabetes mellitus and obesity, are also associated with endothelial dysfunction.9
Previous studies have demonstrated that short periods of inactivity lead to insulin resistance in humans.10–12 We hypothesized that insulin resistance induced by short-term physical inactivity would be associated with vascular dysfunction. This finding would lend further support to basic studies suggesting that insulin resistance and vascular dysfunction share common mechanisms.13 Thus, we assessed vascular function and glucose tolerance before and after a 5-day period of strict bed rest in healthy subjects.
Materials and Methods
Subjects
Healthy nonsmoking volunteers were recruited for this study by newspaper and internet advertisement. Subjects were eligible if they had no clinical history of hypertension, diabetes mellitus, or hyperlipidemia, and were not taking any prescription medications. We sought individuals with preserved flow-dependent vasodilation at baseline, and therefore, excluded subjects if brachial artery flow-mediated dilation was less than 8% at a screening visit. This cut point was selected because it identified a low risk subgroup in a prior prospective study from our laboratory.14 The Institutional Review Board of Boston Medical Center approved the protocol and all subjects provided written informed consent.
Study Protocols
Subjects completed a screening visit to determine eligibility. Up to a month later, they were admitted to the Boston Medical Center General Clinical Research Center for the 5-day study protocol. Subjects were asked to maintain their usual activity level before the study admission. For women, the testing period was scheduled to occur within the follicular period based on previous studies demonstrating stability of vascular function in this phase of the menstrual cycle.15
All subjects began the protocol on a Monday. They arrived in the morning after fasting overnight and had assessment of vascular function and insulin sensitivity, as described below. On completion of baseline studies, subjects began the bed rest period. Subjects remained in bed and were permitted to perform quiet activities such as reading, watching television, and talking on the telephone. Subjects were allotted up to 30 minutes out of bed over the course of each 24-hour period. This time was restricted to activities necessary to perform personal hygiene. A stopwatch was used to monitor all time spent out of bed. The nursing staff monitored subjects to insure adherence to the activity requirements. A clinical nutritionist performed a 24-hour food recall on admission and provided food to match their usual dietary habits. On the third study day (Wednesday), vascular function testing was repeated, and on the fifth study day (Friday), vascular function and assessment of insulin sensitivity were repeated at the same time of day as the baseline evaluation.
As a time and activity control, we assessed vascular function and indices of insulin sensitivity in 9 additional subjects. After baseline testing of vascular function and glucose tolerance, these subjects were instructed to perform their usual level of physical activity. Repeat testing was then performed under the same conditions 5 days later.
Measurement of Vascular Function
Vascular function testing was completed in the morning as previously described.16,17 Endothelium-dependent flow-mediated dilation of the conduit brachial artery was assessed by comparing arterial diameter in 2-dimensional images at baseline and 1 minute after a 5-minute cuff occlusion of the upper arm. After a 10-minute rebaseline period, we assessed endothelium-independent dilation of the same arterial segment before and 4 minutes after sublingual nitroglycerin (0.4 mg). The nitroglycerin portion was omitted if systolic blood pressure was <100 mm Hg or if the patient had prior migraine headaches or adverse reaction to nitroglycerin. Flow-mediated dilation was expressed as the absolute change in diameter in millimeters and as the percent increase in diameter from baseline.
Reactive hyperemia is emerging as a important measure of microvascular function that depends in part on endothelium-derived nitric oxide, is blunted in the setting of cardiovascular risk factors, and relates to cardiovascular events.18–20 We assessed peak reactive hyperemia using Doppler ultrasound to record flow velocity in the brachial artery before and after 5-minute upper arm cuff occlusion. We measured average flow velocity during 5 cardiac cycles immediately after cuff release (peak flow), as previously described.16 Images were digitized, and arterial diameters and Doppler flows were measured with customized software (Medical Imaging Applications, Inc) by individuals blinded to clinical and laboratory status of the subjects.
To gain additional insight into bed rest-induced changes in vascular function, we examined leg blood flow responses using venous occlusion plethysmography in a subset of 10 subjects. Our subjects were allowed to use their arms during the bed rest protocol; thus, vascular function in the lower extremities may have been particularly affected. We also wanted to examine the entire hyperemic response, because the later stages may be more nitric oxide–dependent.21 The leg blood flow measurements were made using a mercury-in-silastic strain gauge placed on the calf, upper thigh and ankle cuffs, and a computerized plethysmograph (Hokanson Inc). During measurements, circulation to the foot was excluded by inflating the ankle cuff to supra-systolic pressure. Five measurements of basal flow were acquired with thigh cuff inflation to 40 mm Hg. After 5 minutes of thigh cuff inflation to supra-systolic pressures, blood flow recordings were made every 15 seconds for a total of 1.5 minutes.
Measurement of Glucose Tolerance
After the vascular function testing, we performed an oral glucose tolerance test.22 Briefly, we measured fasting glucose and insulin levels at baseline and 30, 60, 90, and 120 minutes after the subjects drank a solution containing 75 g of glucose (Trutol, NERL Diagnostics Inc). Net glucose and insulin responses to glucose loading were calculated as the area under the curve over 2 hours. Insulin sensitivity was assessed by 2 validated measures. (1) Homeostasis model assessment-insulin resistance (HOMA-IR) was calculated using the following formula: HOMA-IR=[(fasting insulin μIU/mL)×(fasting glucose mmol/L)]/22.523 and (2) ISI0,120 was calculated as (m/MPG)/log MSI, m is [75,000 mg+(fasting glucose-2-hour glucose)×0.19×body wt (kg)]/120 minutes, MPG is the mean of fasting and 2-hour glucose concentrations (mg/dL), and MSI is the mean of fasting and 2-hour insulin concentrations (mU/L).24
Biochemical Analyses
Total cholesterol, HDL cholesterol, triglycerides, and glucose were measured using enzymatic methods. LDL cholesterol was calculated using the Friedewald formula.25 Insulin was measured using immunochemiluminometric methodology (Quest Diagnostics). Serum C-reactive protein was measured using a high-sensitivity nephelometric method as previously described.26 Serum interleukin (IL)-6, tumor necrosis factor (TNF) receptor-II, and adiponectin were measured using commercially available kits (R&D Systems Inc).
Statistical Analysis
Values are reported as mean±SD unless otherwise specified. Total cholesterol, HDL cholesterol, LDL cholesterol, triglycerides, weight, and glucose were measured on Days 1 and 5 and compared using the paired t test. The Wilcoxon signed rank test was used to compare insulin, HOMA-IR, and C-reactive protein on Days 1 and 5, because these variables did not have a normal distribution based on the Shapiro-Wilk test. Systolic blood pressure, diastolic blood pressure, heart rate, flow-mediated dilation, nitroglycerin mediated dilation, baseline brachial artery diameter, baseline brachial flow, baseline flow velocity, hyperemic flow, hyperemic flow velocity, IL-6, TNF receptor-II, and adiponectin were measured on Days 1, 3, and 5 and compared using repeated-measures analysis of variance (RM-ANOVA). We used 2-way RM-ANOVA to compare the insulin and glucose responses to the oral glucose load as well as the calf hyperemic responses on Days 1 and 5. Statistical significance was accepted at a probability value of <0.05. With the sample size of 20 subjects, the current study had 80% power (alpha=0.05) to detect a change of 22% (1.6 percentage points) in flow-mediated dilation and 15% (170 mL/min) in hyperemic flow, which are comparable to the differences between sedentary and active individuals.27
Results
Baseline Characteristics
Twenty-two healthy subjects, including 14 men and 8 women (mean age, 30.7±8 years) participated in the bed rest protocol. Two subjects (both female) withdrew before completing the protocol. Nine control subjects participated in the normal activity protocol (5 men, 4 women, mean age, 28.8±6.6 years). At baseline, serum cholesterol, insulin, glucose, and systolic and diastolic blood pressure were within normal limits in all subjects.
Physical Inactivity and Glucose Tolerance and Insulin Sensitivity
As shown in Table 1, bed rest increased fasting glucose levels, insulin levels, and HOMA-IR, and decreased ISI0,120. Figure 1 shows the time course of insulin and glucose response to an oral glucose load. The net insulin response(area under the curve) increased by 67% after bed rest compared with baseline (P<0.001 by RM-ANOVA). There was also a 6% increase in the net glucose response (P=0.03 by RM-ANOVA).
Table 1.
Effects of Physical Inactivity on Metabolic Parameters
| Baseline Day 1 | Inactive Day 5 | P Value | |
|---|---|---|---|
| Weight, kg | 70.4±11.4 | 69.9±11.2 | 0.09 |
| Total cholesterol, mg/dL | 147±24 | 159±25 | 0.001 |
| LDL cholesterol, mg/dL | 83±24 | 90±24 | 0.07 |
| HDL cholesterol, mg/dL | 51±12 | 50±11 | 0.38 |
| Triglycerides, mg/dL | 69±21 | 93±30 | 0.001 |
| Glucose, mg/dL | 84.7±7.0 | 87.8±6.1 | 0.02 |
| Insulin, μIU/mL | 4.7±4.1 | 6.9±5.4 | 0.01 |
| HOMA-IR | 1.0±0.9 | 1.5±1.4 | 0.004 |
| ISI0,120, mg*L2/mmol*mIU*min | 32±7 | 28±5 | 0.008 |
Data are presented as mean±SD.
Figure 1.

The effect of bed rest on the insulin (top) and glucose (bottom) response to an oral glucose load in 20 healthy subjects. Bed rest was associated with an increase in the insulin response (P<0.001) and the glucose response (P=0.03), reflecting the development of insulin resistance. Data are mean±SE.
Physical Inactivity and Microvascular Vasodilator Function
Figure 2 displays the effect of bed rest on peak hyperemia flow in the arm measured by Doppler ultrasound. As shown, peak hyperemic response was lower on Day 3 (1,078±268 mL/min, P=0.009 versus baseline) and Day 5 (1113±261 mL/min, P=0.02 versus baseline) of bed rest as compared with the baseline active state (1317±404 mL/min, P=0.01 for overall RM-ANOVA).
Figure 2.
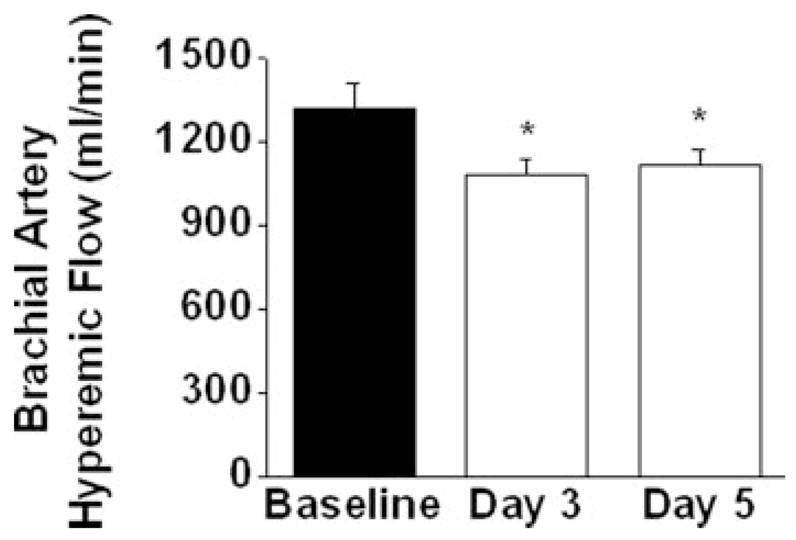
Reactive hyperemia measured by Doppler ultrasound was blunted with bed rest in the upper extremity (P<0.01 for ANOVA, *P<0.05, n=19). Data are mean±SEM.
Figure 3 displays the effect of bed rest on the total hyperemic response in the leg measured by venous occlusion plethysmography. As shown, there was a 29% decrease in total hyperemic response of the leg (P<0.05 by RM-ANOVA). Compared with the active state, peak hyperemic response in the leg also decreased significantly after bed rest (28.5±7.0 mL/min/dL versus 22.2±8.7 mL/min/dL, P=0.003).
Figure 3.
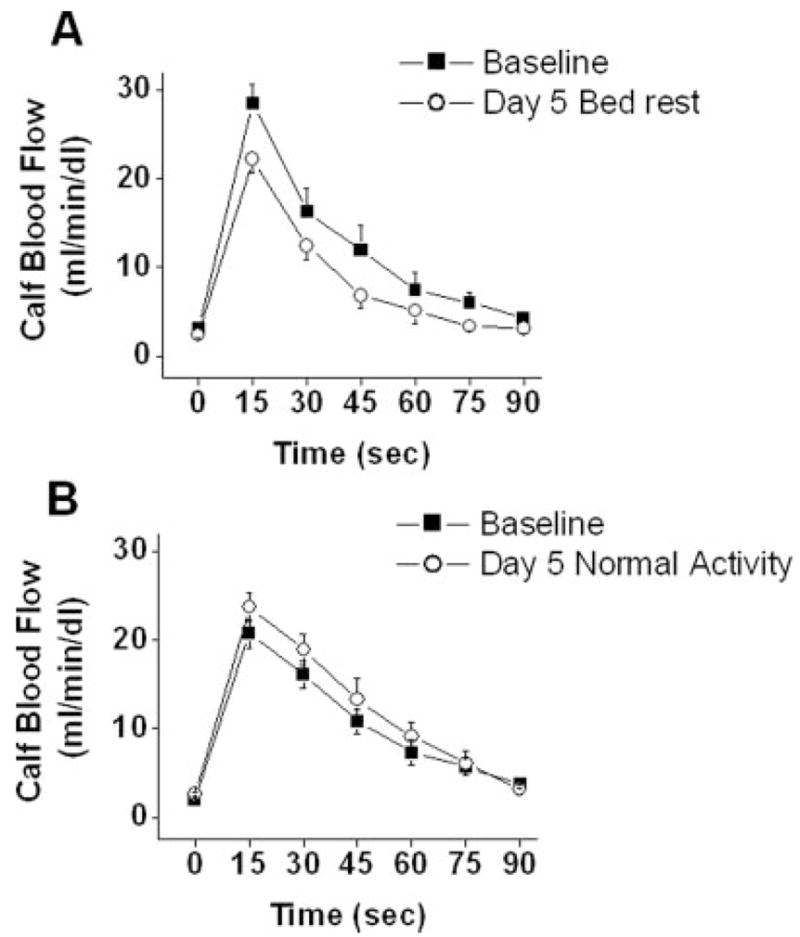
Reactive hyperemia in the lower extremity assessed using venous occlusion plethysmography at baseline and after bed rest (A) or normal activity (B). Bed rest impaired peak and net hyperemic response (*P<0.01 and P<0.05 respectively, n=10). In active control subjects, there were no differences in peak or net hyperemic response (P=NS, n=9).
There were no significant correlations between the bed rest-induced change in reactive hyperemic in the arm and leg and changes in HOMA-IR, ISI0,120, or the insulin response to glucose loading.
As shown in Table 2, there was no significant change in either baseline flow or baseline flow velocity in the brachial artery during bed rest. Baseline flow decreased in the leg after bed rest as measured by plethysmography (2.4±0.9 mL/min/dL versus 3.1±1.2 mL/min/dL in the active state, P=0.02).
Table 2.
Effects of Physical Inactivity on Brachial Artery Parameters and Blood Pressure
| Baseline Day 1 | Bed Rest Day 3 | Bed Rest Day 5 | P Value | |
|---|---|---|---|---|
| Systolic blood pressure, mm Hg | 109±8 | 112±9 | 116±13 | 0.01 |
| Diastolic blood pressure, mm Hg | 64±8 | 66±6 | 68±7 | 0.21 |
| Heart rate, bpm | 64±8 | 64±10 | 64±8 | 0.93 |
| Baseline diameter, mm | 4.19±0.46 | 4.09±0.43 | 4.08±0.46 | 0.02 |
| Flow-mediated dilation, mm | 0.35±0.13 | 0.39±0.12 | 0.35±0.13 | 0.15 |
| Flow-mediated dilation, % | 8.5±3.4 | 9.7±3.4 | 8.7±3.5 | 0.14 |
| Nitroglycerin-mediated dilation, %* | 14.4±3.9 | 19.6±2.9 | 18.9±3.0 | 0.14 |
| Flow-mediated dilation, mm/hyperemic flow velocity, mm* (100* cm/s−1) | 0.23±0.09 | 0.29±0.11 | 0.25±0.10 | 0.003 |
| Baseline flow, mL/min | 199±147 | 153±81 | 156±90 | 0.13 |
| Baseline flow velocity, cm/s | 23.1±2.9 | 18.8±1.6 | 19.3±1.6 | 0.14 |
| Peak flow velocity, cm/s | 157.4±7.4 | 137.4±6.1 | 141.2±5.5 | 0.01 |
Data are presented as mean±SD, P values for RM-ANOVA.
n=7.
Physical Inactivity and Conduit Vessel Vasodilator Function
As shown in Table 2, baseline brachial artery diameter decreased during bed rest as compared with the active state. There was no effect of bed rest on flow-mediated dilation measured as the absolute change or the percent change. Because flow-mediated dilation remained unchanged despite the reduction in hyperemic flow (the stimulus for dilation), we examined the effect of bed rest on the ratio of these 2 variables. As shown in Table 2, this ratio increased significantly during bed rest. Although there also was a tendency for increased nitroglycerin-mediated dilation during bed rest in the subset of patients with available data, this change was not statistically significant, likely reflecting the smaller sample size.
Physical Inactivity and Blood Markers and Hemodynamics
Bed rest was associated with significant increases in total cholesterol and triglycerides (Table 1) and systolic blood pressure (Table 2). There was also a trend toward higher diastolic blood pressure and LDL cholesterol after bed rest. There was no effect of bed rest on body weight, heart rate, or HDL cholesterol. There was no correlation between the bed rest–induced changes in triglyceride levels and markers of insulin resistance including HOMA and the net insulin response (area under the curve).
As shown in Table 3, there was no effect of bed rest on systemic markers of inflammation, including C-reactive protein, IL-6, and TNF receptor-II, and there was no effect on adiponectin levels. There were no significant correlations between the bed rest–induced change in reactive hyperemic and changes in lipids, blood pressure, or markers of inflammation (data not shown).
Table 3.
Effects of Physical Inactivity on Biomarkers
| Baseline Day 1 | Bed Rest Day 3 | Bed Rest Day 5 | P Value | |
|---|---|---|---|---|
| C-reactive protein, mg/L | 1.0±1.2 | … | 1.2±1.9 | 0.40 |
| Interleukin-6, pg/mL | 1.2±0.7 | 1.4±2.2 | 1.0±0.6 | 0.59 |
| Tumor necrosis factor receptor-II, pg/mL | 1870±305 | 1870±370 | 1880±360 | 0.95 |
| Adiponectin, ng/mL | 9540±2950 | 9150±3170 | 9220±3140 | 0.61 |
Data are presented as mean±SD. n=13–19.
Normal Activity and Measures of Vascular Function and Insulin Sensitivity
In the 9 control subjects, there were no changes after 5 days of normal activity in fasting glucose, fasting insulin, insulin AUC, glucose AUC, or lipid parameters (data not shown). Similarly there were no changes in hyperemic flow in the forearm (1005±138 mL/min versus 952±160 mL/min, p=NS) or in the leg (Figure 3). Baseline arterial diameter, baseline flow, flow-mediated dilation, and blood pressure remained unchanged with usual activity (data not shown).
Discussion
This study demonstrated a significant reduction in reactive hyperemia in the upper and lower extremities of healthy volunteers after a short period of bed rest. This impairment of microvascular function was accompanied by a marked increase in the insulin response to an oral glucose load and by increased fasting insulin, glucose levels, and HOMA-IR, changes that are consistent with the development of insulin resistance. Flow-mediated dilation, which reflects vasodilator function of the conduit brachial artery, was not impaired during bed rest, suggesting a preferential impairment of microvascular function. Bed rest was also associated with a decrease in baseline arterial diameter and resting flow and an increase in systolic blood pressure, consistent with increased basal arterial tone and vascular resistance. Furthermore, we observed increases in total cholesterol and triglycerides after bed rest, consistent with the metabolic changes associated with insulin resistance. These changes occurred without changes in systemic inflammatory markers or adiponectin levels. There were no changes observed in vascular function or indices of insulin sensitivity in a control group examined before and after 5 days of usual activity. Collectively, these findings support the hypothesis that physical inactivity concurrently induces a state of insulin resistance and vascular dysfunction in healthy volunteers.
Several lines of evidence support a link between physical inactivity and insulin resistance. Individuals with a sedentary lifestyle have increased risk of developing Type 2 diabetes mellitus.1 Insulin resistance has been shown to develop with prolonged physical inactivity and after only 3 days of bed rest.10 Our study design was based on these observations, and we sought to examine vascular function in a group of previously healthy individuals before and during the earliest stages of insulin resistance.
Several prior studies examined the effect of physical inactivity on microvascular function, but used much more prolonged or restrictive protocols than the present study. For example, decreased reactive hyperemia28 and a decreased blood flow response to acetylcholine infusion29 have been observed after 2 weeks of physical inactivity, but those studies used head-down bed rest, an intervention that produces marked volume shifts that may influence blood flow responses.30 In the present study, vascular dysfunction developed after less than 48 hours, even though subjects were allowed to get out of bed for up to 30 minutes per day. Thus, prolonged periods or extreme forms of physical inactivity are not required, making our observations more relevant to hospitalized patients and sedentary individuals.
Previous studies also examined conduit artery function in the setting of physical inactivity. Consistent with the present study, a decrease in resting femoral artery diameter was observed after prolonged bed rest31 or limb suspension.32 Observational studies have demonstrated impaired conduit artery function in patients with chronic sedentary lifestyle6 or insulin resistance.13 Paradoxically, we observed an increase in flow-mediated dilation when adjusting for the change in reactive hyperemia, and prior investigators have reported similar results.31,32 Because those prior studies also demonstrated an increase in nitroglycerin-mediated dilation, investigators have argued that these findings might reflect increased responsiveness of vascular smooth muscle.31 However, interpretation of the conduit artery responses in this setting is complicated by the concurrent changes in arterial diameter and blood pressure. Smaller baseline arterial size or increased resting arterial tone might lead to proportionally higher flow-mediated dilation that does not reflect improved dilator function. Further studies will be required to determine the mechanisms that account for our observation that short-term bed rest apparently has directionally opposite effects on microvascular and conduit artery vasodilator function.
We considered the possibility that a systemic proinflammatory state might account for impaired microvascular function in our study, because sedentary lifestyle and states of insulin resistance are associated with altered levels of circulating cytokines and adipocytokines.33–35 However, we observed no increase in serum levels of C-reactive protein, IL-6, or soluble TNF receptor II, and no decrease in adiponectin, a protective adipocyte-derived protein.35 Although it remains possible that local inflammation at the level of vascular tissue might have played a role, these findings argue against a systemic state of inflammation or significant alterations in adipocytokine levels as mechanisms for the present findings.
The concurrent development of vascular dysfunction, insulin resistance, increased blood pressure, and dyslipidemia in response to bed rest suggests that these phenomena might share a common mechanism. In cross-sectional studies, states of insulin resistance have been associated with impaired endothelium-dependent vasodilation in peripheral microvessels.36 In the setting of cardiovascular risk factors, including obesity and Type 2 diabetes mellitus, reactive hyperemia is blunted,18,19 reflecting, in part, endothelial dysfunction.18,21 Experimental studies have shown that insulin action and activation of endothelial nitric oxide synthase depend on common signaling mechanisms, including the phosphatidylinositol-3 kinase (PI3 kinase)/Akt system and AMP-dependent protein kinase,13,37 and endothelial nitric oxide synthase null mice display insulin resistance,38 providing further support for a direct link between insulin resistance and vascular dysfunction. Prior studies have also suggested that endothelial dysfunction might contribute to the development of insulin resistance as reduced blood flow led to decreased peripheral insulin-mediated glucose uptake.39 Thus, abnormal glucose tolerance observed with bed rest may be related to reduced peripheral flow, which we observed in the lower extremities of the studied subjects.
A variety of other mechanisms might also contribute to the development of vascular dysfunction during bed rest. For example, the decrease in resting blood flow and the resulting reduction in local shear stress might decrease the expression of endothelial nitric oxide synthase.40 Decreased flow might induce production of reactive oxygen species and endogenous vasoconstrictors that alter vascular function.41 Further studies will be needed to characterize the precise mechanisms accounting for the rapid development of vascular dysfunction and insulin resistance during physical inactivity in humans.
Our study has a number of limitations. First, we cannot exclude the possibility that alterations in diet might have contributed to our findings, although the in-hospital diets were determined by a nutritionist’s assessment. Second, use of a euglycemic clamp would have provided a more precise assessment of insulin sensitivity. Third, the study was limited to noninvasive assessment of vascular function, and our measure of microvascular function was reactive hyperemia. On the other hand, reactive hyperemia is fundamental response of the vasculature that determines tissue perfusion, is impaired with cardiovascular risk factors,19 and predicts cardiovascular events.20 As physical inactivity has multiple potential and observed physiological effects, our study design precludes the establishment of a definitive causal relationship between insulin resistance and vascular dysfunction. The participants were not randomized to normal activity or bed rest; thus, we cannot exclude the possibility that differences between the 2 groups influenced our findings. These limitations are balanced by the relatively large sample size and the marked changes in insulin sensitivity and vascular function in response to a clinically relevant form of physical inactivity.
In summary, we observed the concurrent development of insulin resistance, microvascular dysfunction, dyslipidemia, and increased blood pressure following a short period of bed rest in healthy volunteers. There is a growing epidemic of diseases associated with physical inactivity and insulin resistance including obesity, the metabolic syndrome, and diabetes mellitus.42 Our data are consistent with the hypothesis that insulin resistance has deleterious effects on the vasculature or that common pathophysiological mechanisms account for both effects of bed rest. Our study suggests that the vasculature is highly sensitive to the metabolic changes associated with physical inactivity. On this basis, we speculate that even short periods of inactivity could adversely affect hospitalized patients, and conversely, a program of increased physical activity and exercise might have rapid benefits in previously sedentary patients.
Acknowledgments
Sources of Funding
Dr Hamburg is the recipient of an ACCF/Merck Fellowship and receives support from a Vascular Medicine Training Grant (NIH HL083781). Drs Hamburg, Huang, McMackin, Shenouda, and Widlansky received support from the Boston University School of Medicine Basic Science Cardiovascular Training Program (T32 HL 07224). The work was funded by a Specialized Clinical Center of Research grant from the NIH (P50HL083801), the Boston Medical Center General Clinical Research Center (M01RR00533), and by NIH grants HL081587, and HL083269.
Footnotes
Disclosures
None.
References
- 1.Manson JE, Nathan DM, Krolewski AS, Stampfer MJ, Willett WC, Hennekens CH. A prospective study of exercise and incidence of diabetes among US male physicians. JAMA. 1992;268:63–67. [PubMed] [Google Scholar]
- 2.Blair SN, Kohl HW, III, Paffenbarger RS, Jr, Clark DG, Cooper KH, Gibbons LW. Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA. 1989;262:2395–2401. doi: 10.1001/jama.262.17.2395. [DOI] [PubMed] [Google Scholar]
- 3.Hahn RA, Teutsch SM, Rothenberg RB, Marks JS. Excess deaths from nine chronic diseases in the United States, 1986. JAMA. 1990;264:2654–2659. [PubMed] [Google Scholar]
- 4.Prevalence of physical activity, including lifestyle activities among adults–United States, 2000–2001. MMWR Morb Mortal Wkly Rep. 2003;52:764–769. [PubMed] [Google Scholar]
- 5.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 6.DeSouza CA, Shapiro LF, Clevenger CM, Dinenno FA, Monahan KD, Tanaka H, Seals DR. Regular aerobic exercise prevents and restores age-related declines in endothelium-dependent vasodilation in healthy men. Circulation. 2000;102:1351–1357. doi: 10.1161/01.cir.102.12.1351. [DOI] [PubMed] [Google Scholar]
- 7.Boutcher YN, Boutcher SH. Limb vasodilatory capacity and venous capacitance of trained runners and untrained males. Eur J Appl Physiol. 2005;95:83–87. doi: 10.1007/s00421-005-1377-5. [DOI] [PubMed] [Google Scholar]
- 8.Loscalzo J, Vita JA. Ischemia, hyperemia, exercise, and nitric oxide: Complex physiology and complex molecular adaptations. Circulation. 1994;90:2556–2559. doi: 10.1161/01.cir.90.5.2556. [DOI] [PubMed] [Google Scholar]
- 9.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Jr, Lehman B, Fan S, Osypiuk E, Vita JA. Clinical correlates and heritability of endothelial function in the community: The Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 10.Stuart CA, Shangraw RE, Prince MJ, Peters EJ, Wolfe RR. Bed-rest-induced insulin resistance occurs primarily in muscle. Metabolism. 1988;37:802–806. doi: 10.1016/0026-0495(88)90018-2. [DOI] [PubMed] [Google Scholar]
- 11.Arciero PJ, Smith DL, Calles-Escandon J. Effects of short-term inactivity on glucose tolerance, energy expenditure, and blood flow in trained subjects. J Appl Physiol. 1998;84:1365–1373. doi: 10.1152/jappl.1998.84.4.1365. [DOI] [PubMed] [Google Scholar]
- 12.Smorawinski J, Kaciuba-Uscilko H, Nazar K, Kubala P, Kaminska E, Ziemba AW, Adrian J, Greenleaf JE. Effects of three-day bed rest on metabolic, hormonal and circulatory responses to an oral glucose load in endurance or strength trained athletes and untrained subjects. J Physiol Pharmacol. 2000;51:279–289. [PubMed] [Google Scholar]
- 13.Wheatcroft SB, Williams IL, Shah AM, Kearney MT. Pathophysiological implications of insulin resistance on vascular endothelial function. Diabet Med. 2003;20:255–268. doi: 10.1046/j.1464-5491.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 14.Gokce N, Keaney JF, Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of non-invasively-determined endothelial dysfunction for long-term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003:1769–1775. doi: 10.1016/s0735-1097(03)00333-4. [DOI] [PubMed] [Google Scholar]
- 15.Williams MR, Westerman RA, Kingwell BA, Paige J, Blombery PA, Sudhir K, Komesaroff PA. Variations in endothelial function and arterial compliance during the menstrual cycle. J Clin Endocrinol Metab. 2001;86:5389–5395. doi: 10.1210/jcem.86.11.8013. [DOI] [PubMed] [Google Scholar]
- 16.McMackin CJ, Vita JA. Update on nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2005;396:541–553. doi: 10.1016/S0076-6879(05)960-16-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 18.Higashi Y, Sasaki S, Nakagawa K, Matsuura H, Kajiyama G, Oshima T. A noninvasive measurement of reactive hyperemia that can be used to assess resistance artery endothelial function in humans. Am J Cardiol. 2001;87:121–125. doi: 10.1016/s0002-9149(00)01288-1. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell GF, Parise H, Vita JA, Larson MG, Warner E, Keaney JF, Jr, Keyes MJ, Levy D, Vasan RS, Benjamin EJ. Local shear stress and brachial artery flow-mediated dilation: The Framingham Heart Study. Hypertension. 2004;44:134–139. doi: 10.1161/01.HYP.0000137305.77635.68. [DOI] [PubMed] [Google Scholar]
- 20.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, Shpilman A, Menzoian JO, Watkins MT, Raffetto JD, Gibbons G, Woodson J, Shaw PM, Dhadly M, Eberhardt RT, Keaney JF, Jr, Gokce N, Vita JA. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27:2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meredith IT, Currie KE, Anderson TJ, Roddy MA, Ganz P, Creager MA. Postischemic vasodilation in human forearm is dependent on endothelium-derived nitric oxide. Am J Physiol. 1996;270:H1435–H1440. doi: 10.1152/ajpheart.1996.270.4.H1435. [DOI] [PubMed] [Google Scholar]
- 22.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, Czarnecki EM, Schneiderman N, Skyler JS, Marks JB. Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract. 2000;47:177–184. doi: 10.1016/s0168-8227(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 25.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 26.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 27.Eskurza I, Myerburgh LA, Kahn ZD, Seals DR. Tetrahydrobiopterin Augments Endothelium-Dependent Dilation in Sedentary but Not Habitually Exercising Older Adults. J Physiol. 2005;568:1057–1065. doi: 10.1113/jphysiol.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoemaker JK, Hogeman CS, Silber DH, Gray K, Herr M, Sinoway LI. Head-down-tilt bed rest alters forearm vasodilator and vasoconstrictor responses. J Appl Physiol. 1998;84:1756–1762. doi: 10.1152/jappl.1998.84.5.1756. [DOI] [PubMed] [Google Scholar]
- 29.Hesse C, Siedler H, Luntz SP, Arendt BM, Goerlich R, Fricker R, Heer M, Haefeli WE. Modulation of endothelial and smooth muscle function by bed rest and hypoenergetic, low-fat nutrition. J Appl Physiol. 2005;99:2196–2203. doi: 10.1152/japplphysiol.00888.2005. [DOI] [PubMed] [Google Scholar]
- 30.Zhang LF. Vascular adaptation to microgravity: what have we learned? J Appl Physiol. 2001;91:2415–2430. doi: 10.1152/jappl.2001.91.6.2415. [DOI] [PubMed] [Google Scholar]
- 31.Bleeker MW, De Groot PC, Rongen GA, Rittweger J, Felsenberg D, Smits P, Hopman MT. Vascular adaptation to deconditioning and the effect of an exercise countermeasure: results of the Berlin Bed Rest study. J Appl Physiol. 2005;99:1293–1300. doi: 10.1152/japplphysiol.00118.2005. [DOI] [PubMed] [Google Scholar]
- 32.Bleeker MW, De Groot PC, Poelkens F, Rongen GA, Smits P, Hopman MT. Vascular adaptation to 4 wk of deconditioning by unilateral lower limb suspension. Am J Physiol Heart Circ Physiol. 2005;288:H1747–H1755. doi: 10.1152/ajpheart.00966.2004. [DOI] [PubMed] [Google Scholar]
- 33.Huang AL, Vita JA. Effects of systemic inflammation on endothelium-dependent vasodilation. Trends Cardiovasc Med. 2006;16:15–20. doi: 10.1016/j.tcm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mora S, Lee IM, Buring JE, Ridker PM. Association of physical activity and body mass index with novel and traditional cardiovascular biomarkers in women. JAMA. 2006;295:1412–1419. doi: 10.1001/jama.295.12.1412. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein BJ, Scalia R. Adiponectin: A novel adipokine linking adipocytes and vascular function. Journal of Clinical Endocrinology and Metabolism. 2004;89:2563–2568. doi: 10.1210/jc.2004-0518. [DOI] [PubMed] [Google Scholar]
- 36.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruderman NB, Cacicedo JM, Itani S, Yagihashi N, Saha AK, Ye JM, Chen K, Zou M, Carling D, Boden G, Cohen RA, Keaney J, Kraegen EW, Ido Y. Malonyl-CoA and AMP-activated protein kinase (AMPK): possible links between insulin resistance in muscle and early endothelial cell damage in diabetes. Biochem Soc Trans. 2003;31:202–206. doi: 10.1042/bst0310202. [DOI] [PubMed] [Google Scholar]
- 38.Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes. 2000;49:684–687. doi: 10.2337/diabetes.49.5.684. [DOI] [PubMed] [Google Scholar]
- 39.Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest. 1995;96:786–792. doi: 10.1172/JCI118124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suvorava T, Lauer N, Kojda G. Physical inactivity causes endothelial dysfunction in healthy young mice. J Am Coll Cardiol. 2004;44:1320–1327. doi: 10.1016/j.jacc.2004.06.030. [DOI] [PubMed] [Google Scholar]
- 41.Laufs U, Wassmann S, Czech T, Munzel T, Eisenhauer M, Bohm M, Nickenig G. Physical inactivity increases oxidative stress, endothelial dysfunction, and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:809–814. doi: 10.1161/01.ATV.0000158311.24443.af. [DOI] [PubMed] [Google Scholar]
- 42.Manson JE, Skerrett PJ, Greenland P, VanItallie TB. The escalating pandemics of obesity and sedentary lifestyle. A call to action for clinicians. Arch Intern Med. 2004;164:249–258. doi: 10.1001/archinte.164.3.249. [DOI] [PubMed] [Google Scholar]


